
Sorbonne Université - Faculty of medicine - Saint-Antoine Site – 11th floor
27, rue Chaligny – 75571 Paris cedex 12 - France
Our team studies the molecular and cellular mechanisms of aging and longevity. We seek to better understand the basics of the aging process and how they impact the etiology and progression of age-related diseases, such as Alzheimer's disease, a highly prevalent neurodegenerative disease among elderly subjects.
Our studies focus on the role of insulin-like growth factors IGF-I and -II, and their specific receptor IGF-1R in mammalian longevity. We have shown that IGF signaling not only tightly controls postnatal growth but also very effectively regulates both cell and organism aging. More specifically, we have demonstrated that limiting IGF receptor activation extends lifespan and improves stress resistance(Holzenberger et al. 2003, Xu et al. 2014). We have also shown that functional plasticity of the neuroendocrine somatotropic axis is critical for longevity (Kappeler et al. 2008). Even so, very little is known about the cellular mechanisms by which IGF signaling controls the aging trajectory of different tissues and organs. We have recently demonstrated that suppression of IGF signaling in neural stem cells (NSCs) prevents depletion of neural precursors and maintains the stem cell pool in a youthful state within an aging brain (Chaker et al. 2015, Chaker et al. 2016). These surprising results are backed up by data obtained from mathematical modeling, predicting that a decrease in growth stimulation would indeed be optimal for better tissue aging. In summary, deletion in adult NSCs of the growth and longevity gene IGF-1R induces a gain-of-function phenotype in the aging process, which is marked by optimized cell turnover management, a slender phenotype, and improved olfactory function. These results reveal for the first time that IGF signaling coordinates long-term cell replacement in vivo, and suggest that IGF signaling pathways simultaneously control two major mechanisms of longevity: cell resistance and cell replacement within tissues. We are now expanding this line of research in a new conditional model called UBIKOR (François et al. 2017).
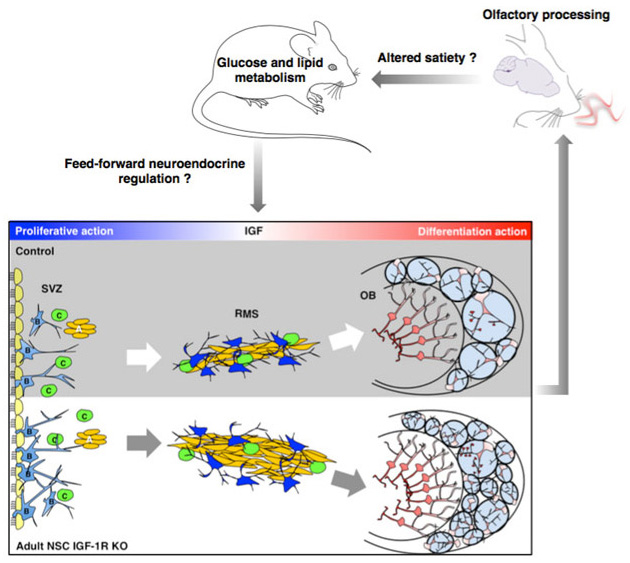 Fig.1 - We induced IGF-1R knockout selectively in adult neuronal stem cells and found that this enhanced long-term neurogenesis in the olfactory cortex. Concomitant with the integration of many additional IGF-1R knockout neurons, olfactory function improved and male mutants progressively developed a prominent metabolic phenotype. Aged mutants were leaner and exerted tighter control over glucose metabolism. In fact, high prevalence of IGF-1R resistant neurons in the adult olfactory bulb mimics effects of food scarcity in an important sensory region of the brain. Consistently, mutants show phenotypic changes typical for systemic adaption to caloric restriction. Question marks indicate hypothetical mechanistic links between OB neurogenesis and metabolic phenotype. More details are in Chaker et al., Aging Cell 2015.
Fig.1 - We induced IGF-1R knockout selectively in adult neuronal stem cells and found that this enhanced long-term neurogenesis in the olfactory cortex. Concomitant with the integration of many additional IGF-1R knockout neurons, olfactory function improved and male mutants progressively developed a prominent metabolic phenotype. Aged mutants were leaner and exerted tighter control over glucose metabolism. In fact, high prevalence of IGF-1R resistant neurons in the adult olfactory bulb mimics effects of food scarcity in an important sensory region of the brain. Consistently, mutants show phenotypic changes typical for systemic adaption to caloric restriction. Question marks indicate hypothetical mechanistic links between OB neurogenesis and metabolic phenotype. More details are in Chaker et al., Aging Cell 2015.
Recently, we have demonstrated that blocking IGF signaling in adult neurons significantly reduces Alzheimer's-like pathology through improved amyloid β peptide clearance (Gontier et al. 2015). Alzheimer's disease (AD) is a common and irreversible neurodegenerative disease with no effective treatment to date. Experimentally, a global decrease in IGF signaling improves symptoms in mice modeling AD (Cohen et al. 2009). We hypothesized that specifically making adult neurons resistant to IGF signaling would protect the aging brain from this fatal neurological disease. To this end, we specifically deleted IGF-1R from adult neurons of transgenic mice modeling AD: as they age, their brains gradually accumulate amyloid plaques associated with neuroinflammation and cognitive deficits. However, Alzheimer’s mutant mice deficient in neuronal IGF-1R exhibit improved spatial memory; their brains have fewer amyloid plaques, fewer amyloid β peptide (Aβ) aggregates, and lower neuroinflammation. For the first time, we established that IGF signaling exerts profound effects on neuronal protein homeostasis and maintenance of cell morphology in vivo (Gontier et al. 2015). At the same time, circulating levels of Aβ are increased. Taken together, our data indicate that neuronal IGF-1R ablation confers lasting protection against AD through enhanced clearance of toxic Aβ, preservation of the autophagic compartment, and improved systemic Aβ elimination. Moreover, functional comparison of transcriptomic profiles of neurons in the early stages of amyloid pathology with IGF-1R-deficient neurons reveals a strong convergence of transcriptomic signatures. These recent data suggest that, in the early stages of the disease, a neuron facing A-proteinopathy succeeds in establishing an endogenous response mimicking that of a neuron lacking IGF-1R (George et al. 2017). Overall, our findings emphasize that the neuronal IGF-1R and its downstream signaling pathways are promising targets for the treatment of AD.
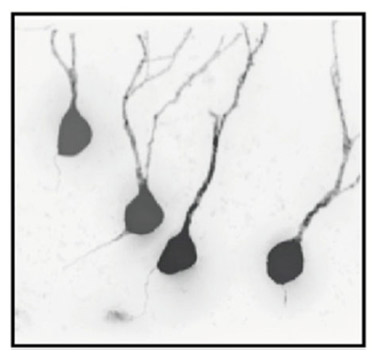
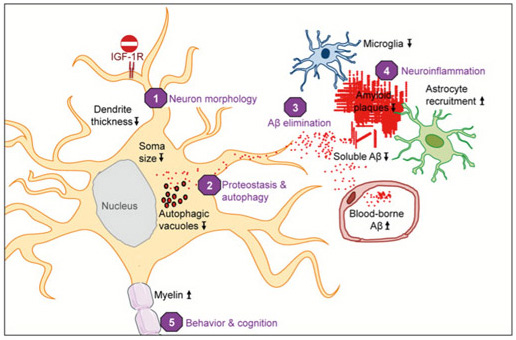 Fig.2 - Neuronal IGF-1R signaling impacts AD progression in multiple ways. Blocking IGF-1R signaling in adult neurons affects cell maintenance and protein homeostasis. Neurons change to a more compact soma and leaner dendrites (1). Autophagy defects in AD, characterized by accumulation of Aβ containing autophagic vacuoles, normalize after IGF-1R inactivation (2). IGF-1R inactivation does not change APP production or processing. Significantly less insoluble Aβ (fewer plaques) and markedly diminished soluble Aβ point to facilitated clearance of toxic peptides from the brain (3). Consequently, neuronal microenvironment is less toxic, as reflected by preserved myelin content and diminished microglial infiltration, possibly preventing less of neurons (4). Cytoarchitectural and functional changes observed after neuronal IGF-1R inactivation in the forebrain of AD improve behavioral and cognitive performances (5). Several different processes are improved in the absence of IGF signaling, suggesting that neuroprotective mechanisms are well adapted to low somatotropic tone.
Fig.2 - Neuronal IGF-1R signaling impacts AD progression in multiple ways. Blocking IGF-1R signaling in adult neurons affects cell maintenance and protein homeostasis. Neurons change to a more compact soma and leaner dendrites (1). Autophagy defects in AD, characterized by accumulation of Aβ containing autophagic vacuoles, normalize after IGF-1R inactivation (2). IGF-1R inactivation does not change APP production or processing. Significantly less insoluble Aβ (fewer plaques) and markedly diminished soluble Aβ point to facilitated clearance of toxic peptides from the brain (3). Consequently, neuronal microenvironment is less toxic, as reflected by preserved myelin content and diminished microglial infiltration, possibly preventing less of neurons (4). Cytoarchitectural and functional changes observed after neuronal IGF-1R inactivation in the forebrain of AD improve behavioral and cognitive performances (5). Several different processes are improved in the absence of IGF signaling, suggesting that neuroprotective mechanisms are well adapted to low somatotropic tone.
In summary, our team has identified IGF signaling pathways as key regulators of multiple interacting processes in the control of aging. We have since focused on (1) the impact of IGF signaling on tissue homeostasis during aging, (2) the neuroprotective mechanisms of IGF resistance in Alzheimer‘s-like neurodegenerative diseases, and (3) the role of growth hormone and IGF signaling in coordinating health and longevity.
 The developmental basis for scaling of mammalian tooth size. Christensen MM, Hallikas O, Das Roy R, Väänänen V, Stenberg OE, Häkkinen TJ, François JC, Asher RJ, Klein OD, Holzenberger M, Jernvall J. Proc Natl Acad Sci USA. (2023) 120 e2300374120. doi: 10.1073/pnas.2300374120.
The developmental basis for scaling of mammalian tooth size. Christensen MM, Hallikas O, Das Roy R, Väänänen V, Stenberg OE, Häkkinen TJ, François JC, Asher RJ, Klein OD, Holzenberger M, Jernvall J. Proc Natl Acad Sci USA. (2023) 120 e2300374120. doi: 10.1073/pnas.2300374120.
https://www.pnas.org/doi/10.1073/pnas.2300374120
 IGF1R suppression in the kidney podocyte has beneficial and detrimental consequences dependent on the level of inhibition. Hurcombe JA, Barrington F, Marchetti M, Betin VMS, Bowen EE, Lay AC, Ni L, Dayalan L, Pope RJP, Brinkkoetter PT, Holzenberger M, Welsh GI, Coward RJM. iScience (2023), accepted manuscript.
IGF1R suppression in the kidney podocyte has beneficial and detrimental consequences dependent on the level of inhibition. Hurcombe JA, Barrington F, Marchetti M, Betin VMS, Bowen EE, Lay AC, Ni L, Dayalan L, Pope RJP, Brinkkoetter PT, Holzenberger M, Welsh GI, Coward RJM. iScience (2023), accepted manuscript.
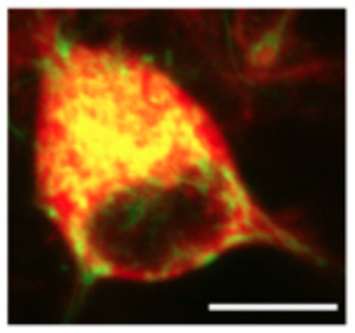 IGF-1 receptor regulates upward firing rate homeostasis via the mitochondrial calcium uniporter. Katsenelson M, Shapira I, Abbas E, Jevdokimenko K, Styr B, Ruggiero A, Aïd S, Fornasiero EF, Holzenberger M, Rizzoli SO, Slutsky I. Proc Natl Acad Sci U S A (2022) 119 e2121040119. doi: 10.1073/pnas.2121040119.
IGF-1 receptor regulates upward firing rate homeostasis via the mitochondrial calcium uniporter. Katsenelson M, Shapira I, Abbas E, Jevdokimenko K, Styr B, Ruggiero A, Aïd S, Fornasiero EF, Holzenberger M, Rizzoli SO, Slutsky I. Proc Natl Acad Sci U S A (2022) 119 e2121040119. doi: 10.1073/pnas.2121040119.
https://www.pnas.org/doi/10.1073/pnas.2121040119
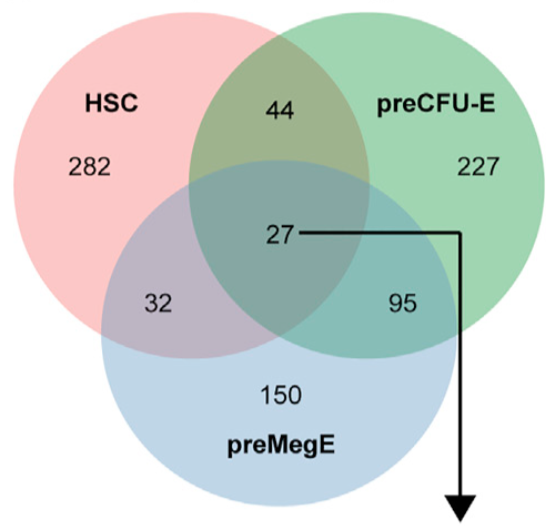 PTBP1 promotes hematopoietic stem cell maintenance and red blood cell development by ensuring sufficient availability of ribosomal constituents. Rehn M, Wenzel A, Frank AK, Schuster MB, Pundhir S, Jørgensen N, Vitting-Seerup K, Ge Y, Jendholm J, Michaut M, Schoof EM, Jensen TL, Rapin N, Sapio RT, Andersen KL, Lund AH, Solimena M, Holzenberger M, Pestov DG, Porse BT. Cell Rep (2022) 39(6):110793. doi: 10.1016/j.celrep.2022.110793.
PTBP1 promotes hematopoietic stem cell maintenance and red blood cell development by ensuring sufficient availability of ribosomal constituents. Rehn M, Wenzel A, Frank AK, Schuster MB, Pundhir S, Jørgensen N, Vitting-Seerup K, Ge Y, Jendholm J, Michaut M, Schoof EM, Jensen TL, Rapin N, Sapio RT, Andersen KL, Lund AH, Solimena M, Holzenberger M, Pestov DG, Porse BT. Cell Rep (2022) 39(6):110793. doi: 10.1016/j.celrep.2022.110793.
https://pubmed.ncbi.nlm.nih.gov/35545054/
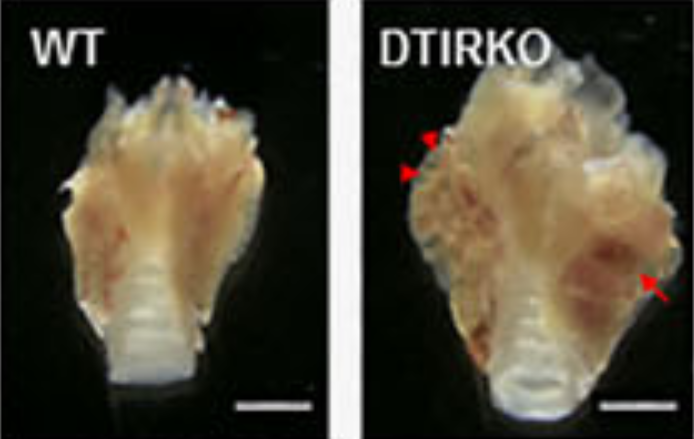 Thyrocyte-specific deletion of insulin and IGF-1 receptors induces papillary thyroid carcinoma-like lesions through EGFR pathway activation. Ock S, Ahn J, Lee SH, Kim HM, Kang H, Kim YK, Kook H, Park WJ, Kim S, Kimura S, Jung CK, Shong M, Holzenberger M, Abel ED, Lee TJ, Cho BY, Kim HS, Kim J. Int J Cancer (2018) 143(10):2458-2469. doi: 10.1002/ijc.31779.
Thyrocyte-specific deletion of insulin and IGF-1 receptors induces papillary thyroid carcinoma-like lesions through EGFR pathway activation. Ock S, Ahn J, Lee SH, Kim HM, Kang H, Kim YK, Kook H, Park WJ, Kim S, Kimura S, Jung CK, Shong M, Holzenberger M, Abel ED, Lee TJ, Cho BY, Kim HS, Kim J. Int J Cancer (2018) 143(10):2458-2469. doi: 10.1002/ijc.31779.
https://pubmed.ncbi.nlm.nih.gov/30070361/
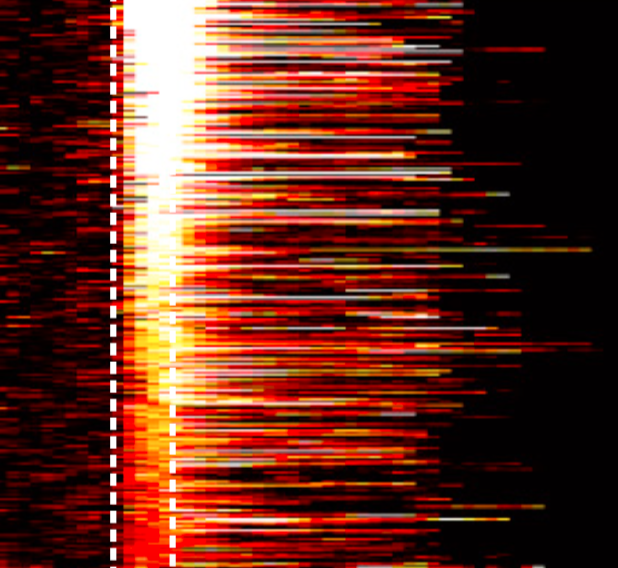 CaMKIIα Expression Defines Two Functionally Distinct Populations of Granule Cells Involved in Different Types of Odor Behavior. Malvaut S, Gribaudo S, Hardy D, David LS, Daroles L, Labrecque S, Lebel-Cormier MA, Chaker Z, Coté D, De Koninck P, Holzenberger M, Trembleau A, Caille I, Saghatelyan A. Curr Biol (2017) 27(21):3315-3329.e6. doi: 10.1016/j.cub.2017.09.058.
CaMKIIα Expression Defines Two Functionally Distinct Populations of Granule Cells Involved in Different Types of Odor Behavior. Malvaut S, Gribaudo S, Hardy D, David LS, Daroles L, Labrecque S, Lebel-Cormier MA, Chaker Z, Coté D, De Koninck P, Holzenberger M, Trembleau A, Caille I, Saghatelyan A. Curr Biol (2017) 27(21):3315-3329.e6. doi: 10.1016/j.cub.2017.09.058.
https://pubmed.ncbi.nlm.nih.gov/29107547/
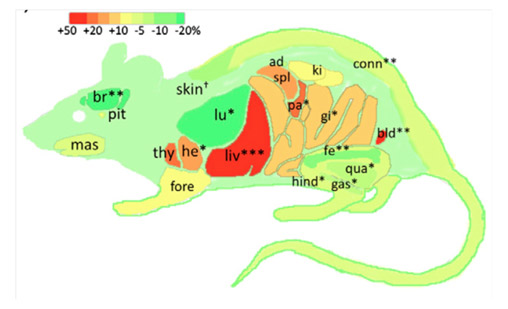 Disrupting IGF Signaling in Adult Mice Conditions Leanness, Resilient Energy Metabolism, and High Growth Hormone Pulses. François JC, Aïd S, Chaker Z, Lacube P, Xu J, Fayad R, Côté F, Even P, Holzenberger M. Endocrinology (2017) 158: 2269-2283.
Disrupting IGF Signaling in Adult Mice Conditions Leanness, Resilient Energy Metabolism, and High Growth Hormone Pulses. François JC, Aïd S, Chaker Z, Lacube P, Xu J, Fayad R, Côté F, Even P, Holzenberger M. Endocrinology (2017) 158: 2269-2283.
https://academic.oup.com/endo/article/158/7/2269/3813249
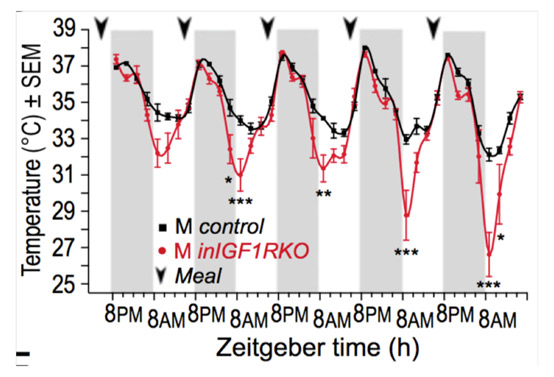 Insulin-like growth factor 1 receptor regulates hypothermia during calorie restriction. Cintron-Colon R, Sanchez-Alavez M, Nguyen W, Mori S, Gonzalez-Rivera R, Lien T, Bartfai T, Aïd S, François JC, Holzenberger M, Conti B. Proc Natl Acad Sci U S A (2017) 114: 9731-9736.
Insulin-like growth factor 1 receptor regulates hypothermia during calorie restriction. Cintron-Colon R, Sanchez-Alavez M, Nguyen W, Mori S, Gonzalez-Rivera R, Lien T, Bartfai T, Aïd S, François JC, Holzenberger M, Conti B. Proc Natl Acad Sci U S A (2017) 114: 9731-9736.
https://www.pnas.org/content/114/36/9731
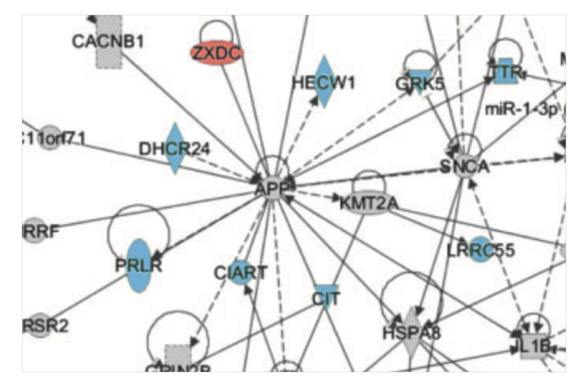 The Alzheimer's disease transcriptome mimics the neuroprotective signature of IGF-1 receptor-deficient neurons. George C, Gontier G, Lacube P, François JC, Holzenberger M, Aïd S. Brain (2017) 140: 2012-2027.
The Alzheimer's disease transcriptome mimics the neuroprotective signature of IGF-1 receptor-deficient neurons. George C, Gontier G, Lacube P, François JC, Holzenberger M, Aïd S. Brain (2017) 140: 2012-2027.
https://academic.oup.com/brain/article/140/7/2012/3864005
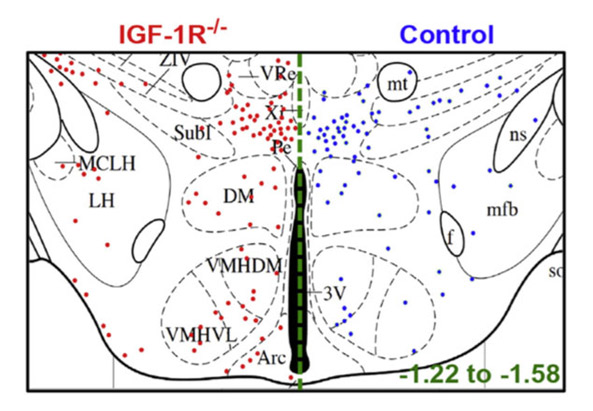 Hypothalamic neurogenesis persists in the aging brain and is controlled by energy-sensing IGF-I pathway. Chaker Z, Petrovska M, Caron JB, Lacube P, Caillé I, Holzenberger M.. Neurobiol Aging (2016) 41: 64-72.
Hypothalamic neurogenesis persists in the aging brain and is controlled by energy-sensing IGF-I pathway. Chaker Z, Petrovska M, Caron JB, Lacube P, Caillé I, Holzenberger M.. Neurobiol Aging (2016) 41: 64-72.
https://www.sciencedirect.com/science/article/abs/pii/S0197458016001512?via%3Dihub
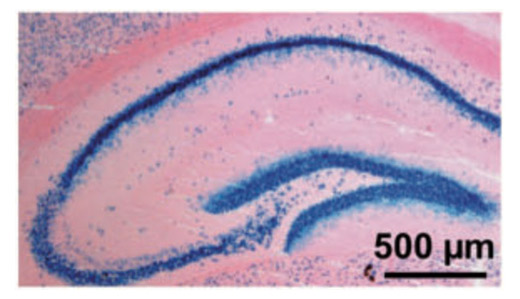 Deleting IGF-1 receptor from forebrain neurons confers neuroprotection during stroke and upregulates endocrine somatotropin. De Magalhaes Filho CD, Kappeler L, Dupont J, Solinc J, Villapol S, Denis C, Nosten-Bertrand M, Billard JM, Blaise A, Tronche F, Giros B, Charriaut-Marlangue C, Aïd S, Le Bouc Y, Holzenberger M. J Cerebr Blood Flow Metab (2017) 37: 396-412.
Deleting IGF-1 receptor from forebrain neurons confers neuroprotection during stroke and upregulates endocrine somatotropin. De Magalhaes Filho CD, Kappeler L, Dupont J, Solinc J, Villapol S, Denis C, Nosten-Bertrand M, Billard JM, Blaise A, Tronche F, Giros B, Charriaut-Marlangue C, Aïd S, Le Bouc Y, Holzenberger M. J Cerebr Blood Flow Metab (2017) 37: 396-412.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5381438/
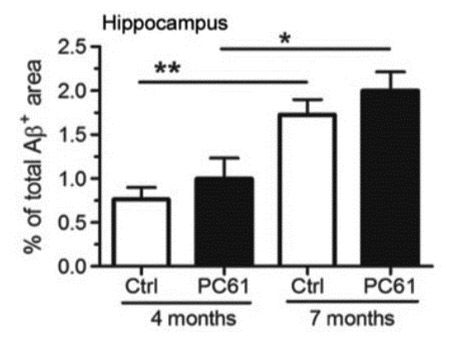 Regulatory T cells delay disease progression in Alzheimer-like pathology. Dansokho C, Ait Ahmed D, Aïd S, Toly-Ndour C, Chaigneau T, Calle V, Cagnard N, Holzenberger M, Piaggio E, Aucouturier P, Dorothée G. Brain (2016) 139: 1237-1251.
Regulatory T cells delay disease progression in Alzheimer-like pathology. Dansokho C, Ait Ahmed D, Aïd S, Toly-Ndour C, Chaigneau T, Calle V, Cagnard N, Holzenberger M, Piaggio E, Aucouturier P, Dorothée G. Brain (2016) 139: 1237-1251.
https://academic.oup.com/brain/article/139/4/1237/2464189
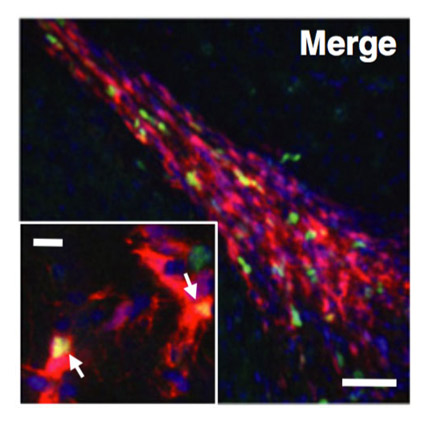
 Blocking IGF signaling in adult neurons alleviates Alzheimer’s disease pathology through amyloid-β clearance. Gontier G, George C, Chaker Z, Holzenberger M, Aïd S. J Neurosci (2015) 35: 11500-11513.
Blocking IGF signaling in adult neurons alleviates Alzheimer’s disease pathology through amyloid-β clearance. Gontier G, George C, Chaker Z, Holzenberger M, Aïd S. J Neurosci (2015) 35: 11500-11513.
http://www.jneurosci.org/content/35/33/11500.long
 Suppression of IGF-I signals in neural stem cells enhances neurogenesis and olfactory function during aging. Chaker Z, Aïd S, Berry H, Holzenberger M. Aging Cell (2015) 14: 847-856.
Suppression of IGF-I signals in neural stem cells enhances neurogenesis and olfactory function during aging. Chaker Z, Aïd S, Berry H, Holzenberger M. Aging Cell (2015) 14: 847-856.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4568972/
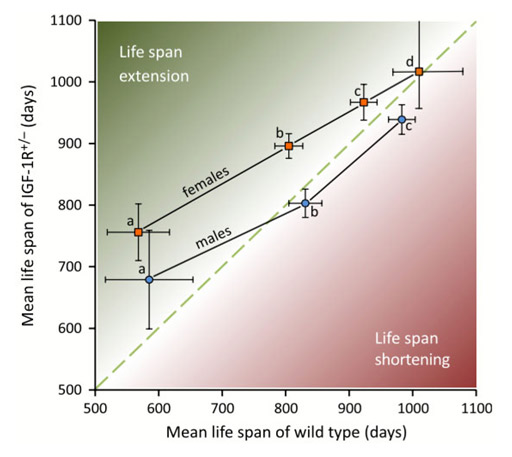 Longevity effect of IGF-1R+/- mutation depends on genetic background-specific receptor activation. Xu J, Gontier G, Chaker Z, Lacube P, Dupont J, Holzenberger M. Aging Cell (2014) 13: 19-28.
Longevity effect of IGF-1R+/- mutation depends on genetic background-specific receptor activation. Xu J, Gontier G, Chaker Z, Lacube P, Dupont J, Holzenberger M. Aging Cell (2014) 13: 19-28.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4326867/
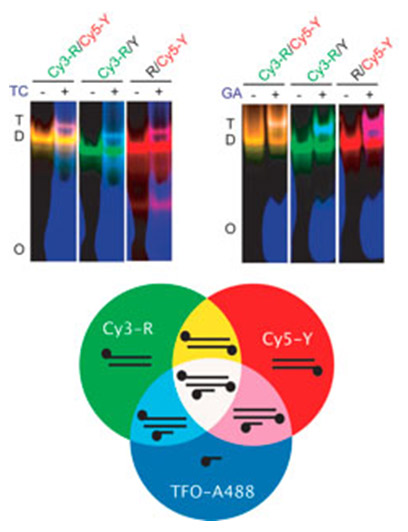 Monitoring DNA triplex formation using multicolor fluorescence and application to insulin-like growth factor I promoter downregulation. Hégarat N, Novopashina D, Fokina AA, Boutorine AS, Venyaminova AG, Praseuth D, François JC. FEBS J (2014) 281: 1417-1431.
Monitoring DNA triplex formation using multicolor fluorescence and application to insulin-like growth factor I promoter downregulation. Hégarat N, Novopashina D, Fokina AA, Boutorine AS, Venyaminova AG, Praseuth D, François JC. FEBS J (2014) 281: 1417-1431.
http://www.onlinelibrary.wiley.com/doi/10.1111/febs.12714/full
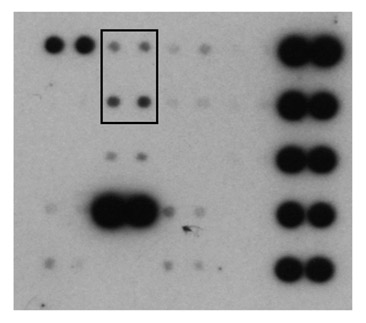 Small interfering RNA targeted to IGF-IR delays tumor growth and induces proinflammatory cytokines in a mouse breast cancer model. Durfort T, Tkach M, Meschaninova MI, Rivas MA, Elizalde PV, Venyaminova AG, Schillaci R, François JC. PLoS ONE (2012) 7: e29213.
Small interfering RNA targeted to IGF-IR delays tumor growth and induces proinflammatory cytokines in a mouse breast cancer model. Durfort T, Tkach M, Meschaninova MI, Rivas MA, Elizalde PV, Venyaminova AG, Schillaci R, François JC. PLoS ONE (2012) 7: e29213.
http://www.journals.plos.org/plosone/article?id=10.1371/journal.pone.0029213
 Exploring endocrine GH pattern in mice using rank plot analysis and random blood samples. Xu J, Bekaert AJ, Dupont J, Rouve S, Annesi-Maesano I, De Magalhaes Filho CD, Kappeler L, Holzenberger M. J Endocrinol (2011) 208: 119-129.
Exploring endocrine GH pattern in mice using rank plot analysis and random blood samples. Xu J, Bekaert AJ, Dupont J, Rouve S, Annesi-Maesano I, De Magalhaes Filho CD, Kappeler L, Holzenberger M. J Endocrinol (2011) 208: 119-129.
https://joe.bioscientifica.com/view/journals/joe/208/2/119.xml
 Early postnatal nutrition determines somatotropic function in mice. Kappeler L, De Magalhaes Filho C, Leneuve P, Xu J, Brunel N, Chatziantoniou C, Le Bouc Y, Holzenberger M. Endocrinology (2009) 150: 314-323.
Early postnatal nutrition determines somatotropic function in mice. Kappeler L, De Magalhaes Filho C, Leneuve P, Xu J, Brunel N, Chatziantoniou C, Le Bouc Y, Holzenberger M. Endocrinology (2009) 150: 314-323.
https://academic.oup.com/endo/article/150/1/314/2455886
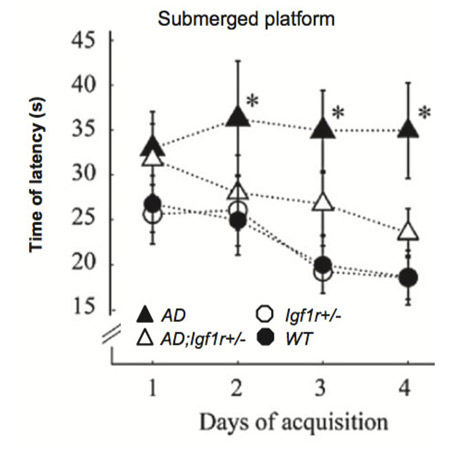 Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, Masliah E, Dillin A. Cell (2009) 139: 1157-1169.
Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, Masliah E, Dillin A. Cell (2009) 139: 1157-1169.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3017511/
 Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. Kappeler L, De Magalhaes FilhoC, Dupont J, LeneuveP, Cervera P, PérinL, Loudes C, Blaise A, Klein R, Epelbaum J, Le BoucY, Holzenberger M. PLoS Biol (2008)6: 2144-2153.
Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. Kappeler L, De Magalhaes FilhoC, Dupont J, LeneuveP, Cervera P, PérinL, Loudes C, Blaise A, Klein R, Epelbaum J, Le BoucY, Holzenberger M. PLoS Biol (2008)6: 2144-2153.
http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.0060254
INSERM
Kourilsky Building
34 rue Crozatier - 75012 Paris
France
Sorbonne Université Medicine
Saint-Antoine Site
27 rue Chaligny - 75012 Paris
France
