
Saint-Antoine Hospital - Kourilsky Building – 5th floor –
184, rue du Faubourg Saint-Antoine - 75012 Paris - France
Rheumatology at Saint-Antoine: LaRhumato.fr
Research on osteoarthritis
Osteoarthritis, a disease affecting mainly the peripheral joints and the spine, affects 6 million people in France, with a worldwide prevalence of the disease recently estimated at more than 250 million people. Globally, peripheral/spinal osteoarthritis is the most common cause of disability with annual medical expenses exceeding €3.4 billion, a doubling in 10 years. The main symptom of osteoarthritis is pain, which can involve different mechanisms (nociceptive pain, neuropathic pain, central sensitization). This symptom remains poorly understood, especially since it may be partly imperfectly correlated with articular tissue alterations.
Unfortunately, the only clinically recognized treatment strategies are symptomatic, and there are no approved structural drugs for OA. Effective treatment of OA is therefore a critical unmet need.
Our team includes physiologists, cell biologists, biochemists, bioinformaticians, rheumatologists and orthopedic surgeons who are dedicated to the search for new therapeutic targets for OA. For this purpose, we take advantage of recent knowledge on the pathophysiology of the disease:
• Osteoarthritis was initially considered to be a cartilage-only disease. We now know that OA is a much more complex disease, involving not only cartilage, but also bone and synovial tissue. Indeed, studies suggest that the structural progression of OA may result from altered communication between subchondral bone cells (e.g., osteoblasts, osteocytes, endothelial cells, adipocytes), chondrocytes, and synoviocytes.
• Given that obesity and cardiometabolic diseases are risk factors for OA not only in weight-bearing joints but also in the hands, systemic mediators and low-grade systemic inflammation certainly play a critical role in the initiation and/or aggravation of OA and in pain. This inflammation can be modulated by environmental factors, gut microbiota and the autonomic nervous system
Based on these recent findings, our main projects currently under study are:
• To find new mediators involved in the disrupted communication between cartilage and subchondral bone in order to modulate their expression for therapeutic purposes.
• Determine the role of inflammatory/metabolic/external neural signals on intertissular communication defects that could be manipulated for therapeutic purposes
• To determine the molecular actors involved in joint pain, the main symptom of osteoarthritis
• To study the role of environmental factors such as endocrine disruptors in the genesis of OA .
Methodologies used :
• Experimental models of osteoarthritis (mice) (collagenase, meniscal destabilization, aging)
• Primary culture of articular chondrocytes (mouse, human) and costal chondrocytes (mouse), human and murine osteoblasts (3D membrane), human synoviocytes and mesenchymal stem cells (from bone marrow and human and rat adipose tissue).
• Control of chondrocyte phenotype (hypertrophic differentiation and fibroblastic de-differentiation)
• Bone/cartilage communication model (murine)
• Differentiation of mesenchymal stem cells into chondrocytes, osteoblasts and adipocytes
• Cellular tests (proliferation, adhesion, angiogenesis)
• Cellular analysis (Western-blot, proteolytic activity, ELISA, qRT-PCR, RNA interference, immunocytology)
• BioJoint biobank of human arthrosic tissues for tissue, cellular, biochemical and molecular analysis
• Cohort of osteoarthritis patients followed longitudinally with collection of clinical, biological and imaging data
• Tissue analysis (histology, immunohistochemistry)
• Bioinformatics analysis
Most importants facts
1 - Finding new mediators involved in the disturbed communication between cartilage and subchondral bone with the aim of modulating their expression for therapeutic purposes.
There is a profound remodeling of the osteochondral junction during osteoarthritis, leading to the disappearance of articular cartilage and sclerosis of the subchondral bone. This remodeling can be explained by a pathological reactivation of the endochondral ossification process which includes hypertrophic differentiation of chondrocytes, cartilage mineralization, increased angiogenesis and resorption followed by bone synthesis.
The project is interested in determining the cellular and molecular actors at the origin of this pathological remodeling. In the cartilage component, we are interested in the hypertrophic differentiation of chondrocytes; in the factors involved in this differentiation (collaborative project financed by the SFR) and in those produced by hypertrophic chondrocytes which could stimulate angiogenesis and osteoclastogenesis (ARDoC financing of I. Toillon's thesis). In the bone component, we are studying the consequences of excessive mechanical stress on the angiogenic activity of osteoblasts. We are also studying the role that medullary adipose tissue cells (adipocytes and mesenchymal stromal cells) could play (SFR Network funding and FRM funding of N. Zapata's postdoc). Our published data (Eymard, Arthritis Rheumatol 2014; Eymard, Ann Rheum Dis 2017) and in progress suggest that intra-articular adipose tissues are new tissue actors involved in osteoarthritis (Ramsay Générale de Santé funding). Finally, we are developing in collaboration with a chemist (Dr. J. Landoulsi) a new approach by atomic force microscopy to analyze the structural modifications of the osteochondral junction in osteoarthritis (IPV - Sorbonne University funding of I. Jaabar's thesis).
Protein 14-3-3 : Recently, studies have shown the involvement of innate immunity and inflammation in the progression of osteoarthritis. Our team has identified the 14-3-3ε protein as a new catabolic factor, produced by the subchondral bone and able to induce a catabolic phenotype on chondrocytes. We found that these effects are mediated, at least in part, by signaling via TLR2 or TLR4 receptors, leading to activation of innate immunity as alarmins do. Furthermore, it is established that alarmines can stimulate the activation of resident immune cells of the synovial membrane, in particular macrophages leading to synovial inflammation. We were able to show that the polarization of macrophages stimulated by 14-3-3ε seems to direct them towards an M1 inflammatory phenotype. Thus, our results point to 14-3-3ε as a novel alarmin in OA, triggering catabolic and inflammatory effects via TLR signaling. This novel candidate could represent a new target for therapeutic and/or prognostic purposes.
Identification of soluble 14-3-3 as a novel subchondral bone mediator involved in cartilage degradation in osteoarthritis. S. Priam, C. Bougault, X. Houard, C. Salvat, M. Gosset, F. Berenbaum and C. Jacques.Arthritis and Rheumatism. 2013 Jul;65(7):1831-42.
The pro-inflammatory cytokine s14-3-3ε is a ligand of CD13/Aminopeptidase N in cartilage. Nefla M, Sudre L, Denat G, Priam S, Andre-Leroux G, Berenbaum F, Jacques C. J Cell Sci. 2015 Sep 1;128(17):3250-62.
The danger from within: alarmins in arthritis. Nefla M, Holzinger D, Berenbaum F and Jacques C.Nat Rev Rheumatol. 2016 12(11):669- 683.
Knee and hip intra-articular adipose tissues (IAATs): a common phenotype for a central player in osteoarthritis. Eymard F, Pigenet A, Citadelle D, Tordjman J, Foucher L, Rose C, Flouzat Lachaniette CH, Clément K, Berenbaum F, Chevalier X, Houard X. Ann. Rheum Dis. 2017;76(6):1142-48.
Infrapatellar fat pad induces an inflammatory and pro-degradative phenotype on autologous fibroblast-like synoviocytes from severe knee osteoarthritis patients. Eymard F, Pigenet A, Citadelle D, Flouzat-Lachaniette CH, Poignard A, Benelli C, Berenbaum F, Chevalier X, Houard X. Arthritis Rheumatol. 2014; 66(8):2165-74.
2 - Determine the role of the cholinergic system and its manipulation for therapeutic purposes.
We also take an integrative view of the pathophysiology of OA. In essence, we are studying the role of the autonomic nervous system (and in particular of its main mediator, acetylcholine) in the pathophysiology of osteoarthritis and joint inflammation. Using a recent method that employs a 3D immunolabeling protocol of the whole joint, we have demonstrated cholinergic innervation of joint tissues (subchondral bone and synovium) in human and murine joint samples (collaboration with Alain CHEDOTAL Institut de la Vision, INSERM, Paris, France). We are also investigating whether chondrocytes and osteoblasts are able to produce acetylcholine themselves (corresponding to a non-neuronal production of acetylcholine) and whether the acetylcholine/acetylcholine receptor axis (alpha-7 nicotinic receptor (Chrna7)) can regulate joint inflammation 7 (collaboration with Uwe MASKOS, Unit of Integrative Neurobiology of Cholinergic Systems, CNRS UMR 3571, Institut Pasteur, Paris, France). We have shown that the acetymholin/alpha-7 nicotinic receptor axis modulates the inflammatory response and the degradation of joint tissues. This has led us to try the auricular stimulation of the vagus nerve in painful and inflammatory hand osteoarthritis in the framework of a PHRC.
Human-specific duplicate CHRFAM7A gene is associated with more severe osteoarthritis and amplifies pain behaviors. Courties A, Olmer M, Myers K, Ordoukhanian P, Head SR, Natarajan P, Berenbaum F, Sellam J, Lotz MK.Ann Rheum Dis. 2023 Jan 10:ard-2022-223470.
Alpha-7 Nicotinic Receptor Dampens Murine Osteoblastic Response to Inflammation and Age-Related Osteoarthritis. Courties A, Petit J, Do A, Legris M, Kouki I, Pigenet A, Sacitharan PK, Ehkirch FP, Berenbaum F, Sellam J. Front Immunol. 2022 Apr 8;13:842538.
The Role of the Non-neuronal Cholinergic System in Inflammation and Degradation processes in Osteoarthritis. Courties A, Do A, Leite S, Legris M, Sudre L, Pigenet A, Petit J, Nourissat G, Cambon-Binder A, Maskos U, Berenbaum F, Sellam J. Arthritis Rheumatol. 2020 Jul 8. doi: 10.1002/art.41429. Online ahead of print.
Clearing method for 3-dimensional immunofluorescence of osteoarthritic subchondral human bone reveals peripheral cholinergic nerves. Courties A, Belle M, Senay S, Cambon-Binder A, Sautet A, Chédotal A, Berenbaum F, Sellam J. Sci Rep. 2020 Jun 1;10(1):8852.
Role of the autonomic nervous system in osteoarthritis. Courties A, Sellam J, Berenbaum F. Best Pract Res Clin Rheumatol. 2017 Oct;31(5):661-675
At the cellular level:
• Primary culture of articular chondrocytes (mouse, human) and costal chondrocytes (mouse), human and murine osteoblasts (3D membrane), human synoviocytes, macrophages, mesenchymal stem cells (from bone marrow and human and rat adipose tissue), endothelial cells
• Control of chondrocyte phenotype (hypertrophic differentiation and fibroblastic de-differentiation)
• Cellular tests (proliferation, adhesion, angiogenesis)
• Cellular analysis (Western-blot, proteolytic activity, ELISA, qRT-PCR, RNA interference, immunocytology)
Tissue level:
• Cartilage explant culture, histology, immunohistochemistry , biochemistry and molecular analysis of joint tissue.
In vivo models:
• Experimental model of osteoarthritis in mice and rats (post-traumatic, monoiodoacetate, collagenase)
Human data:
• BioJoint: Biobank of human osteoarthritis tissues (cartilage, bone, synovial tissue and fluid, adipose tissue) for tissue, cellular, biochemical and molecular analyses.
• DIGICOD: a cohort of 426 patients with hand osteoarthritis (clinical phenotyping, DNA, serum, X-rays, MRI, ultrasound)
• TRANSIMMUNOM: a cohort of patients with inflammatory diseases including 50 knee osteoarthritis.
International collaborations :
• Hong Kong (Polytechnic University)
• San Diego (Scripps Institute)
• San Francisco (UCSF Bakar Institute)
Keywords :
• osteoarthritis
• inflammation
• adipose tissue
• cholinergic system
• cartilage
• synovial tissue
• subchondral bone
• mouse models of osteoarthritis
• in vitro/ex vivo culture of joint cells
• chondrocyte differentiation models
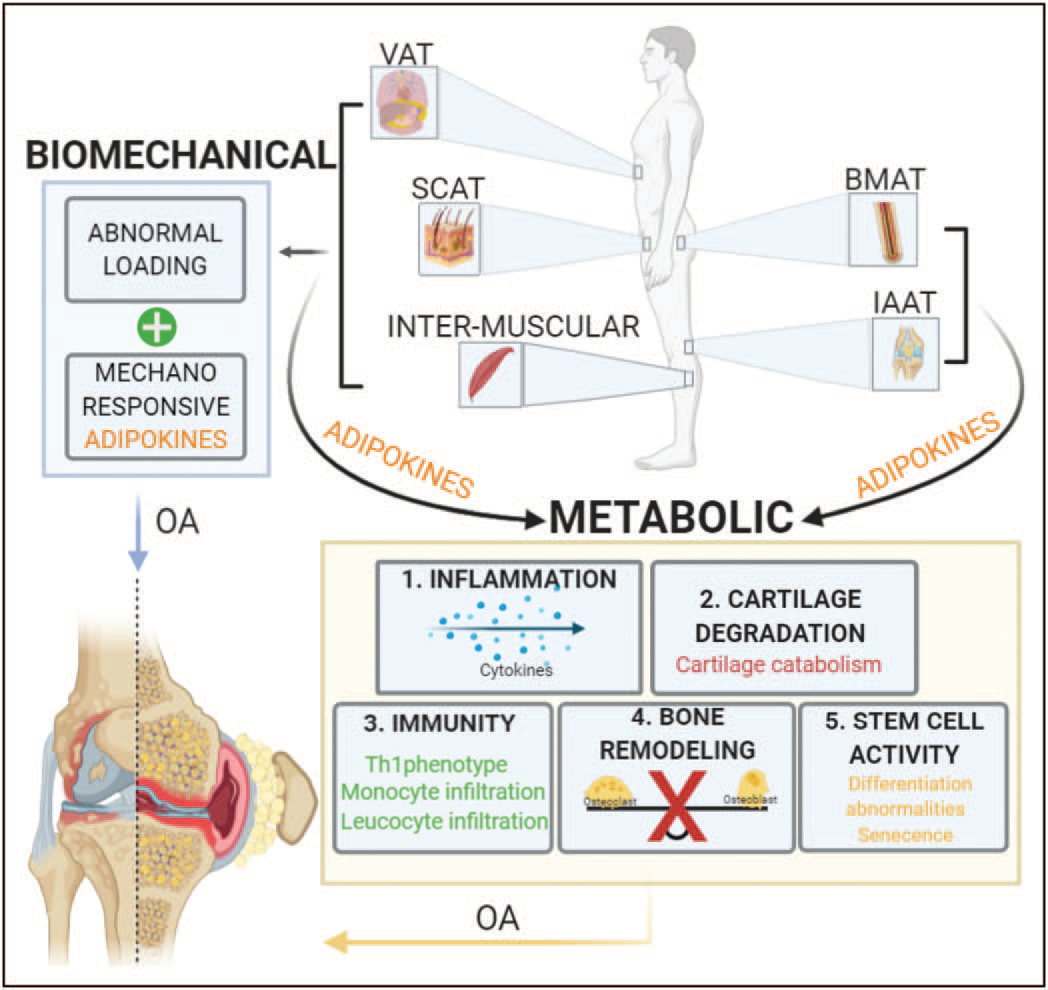
Roles of the different adipose tissues on osteoarthritis progression by biomechanical and metabolic mechanisms. Increases on systemic adipose tissues like subcutaneous adipose tissue, visceral adipose tissue and intra-muscular adipose tissue contribute to abnormal loading of the joint, this mechanical stress have been shown to be part of osteoarthritis onset and progression. Lipocalin adipokine family has emerged as sensors of mechanical load, inflammatory status and catabolic stimuli of the joint, suggesting its involvement in osteoarthritis pathophysiology. On the other hand, the paracrine role of subcutaneous adipose tissue, visceral adipose tissues, intra muscular adipose tissues and local adipose tissues bone marrow adipose tissue and intra-articular adipose tissue affect joint health. The adipokines secreted by all those tissues have proven to promote directly: 1. secretion of inflammatory cytokines like IL-1b and TNF-a which are well documented for their active involvement in the pathophysiology of osteoarthritis, 2. cartilage catabolism, including inhibition of proliferation in chondrocytes and degradation of the matrix components, collagen type 2 and agrecan, 3. Immune response by the infiltration of joint tissues by monocytes and leukocytes which increases even more the inflmmatory signals present on the affected joint, 4. Loss of balance between osteoclast and osteoblast affecting directly bone remodelling, changes on bone constitution are part of osteoarthritis pathophysiology and 5. Changes on stem-cell principal characteristics like prolifereation and differentiation capacity.
From Curr Opin Rheumatol 2021, 33:84-93
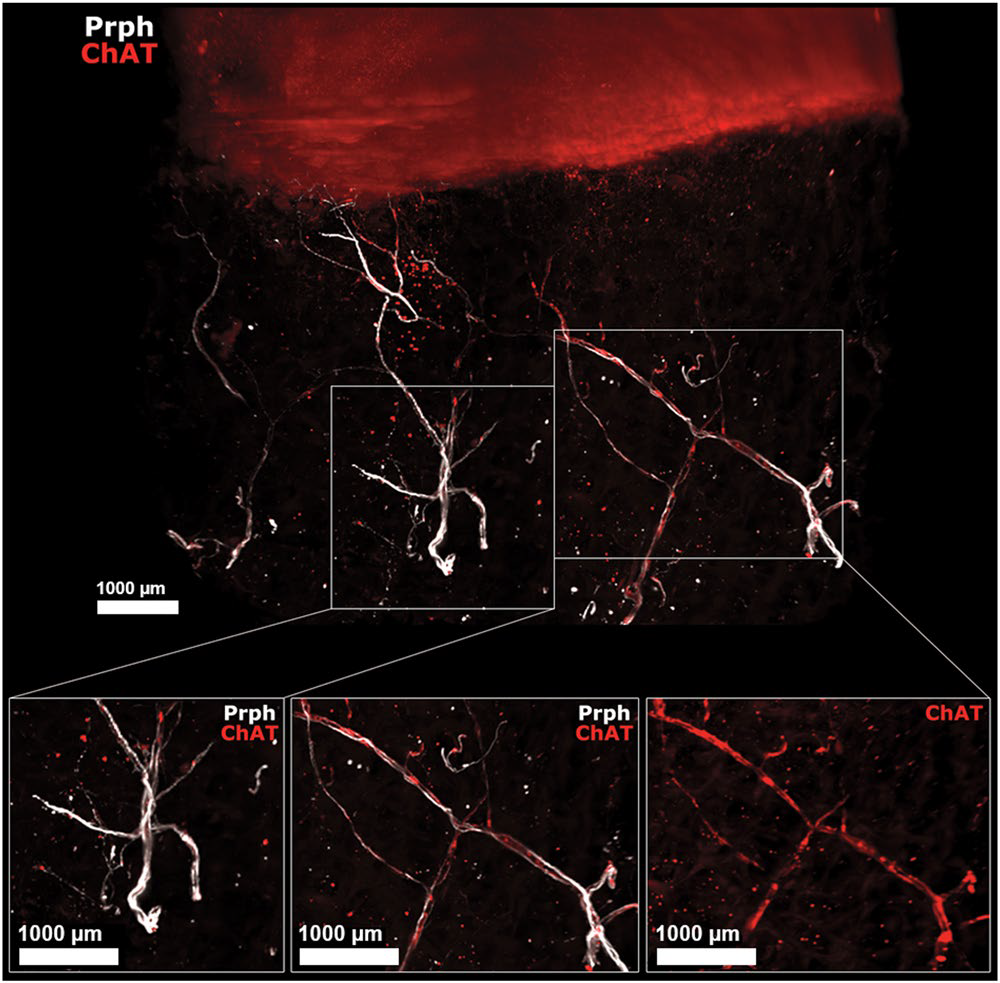
Human OA subchondral bone has peripheral and cholinergic nerves. Immunofluorescence of peripherin and ChAT in human OA subchondral bone (see video 2) after the 3DISCO clearing protocol and analysis with Imaris. The colocalization of both markers showed that some subchondral bone peripheral nerves marked by Prph (white) are also cholinergic, as they showed ChAT immunofluorescence (red) as shown on the right while some did not expressed ChAT (left). ChAT: choline acetyltransferase, Prph: peripherin.
From Scientific Reports | (2020) 10:8852
INSERM
Kourilsky Building
34 rue Crozatier - 75012 Paris
France
Sorbonne Université Medicine
Saint-Antoine Site
27 rue Chaligny - 75012 Paris
France
