
Sorbonne Université - Faculty of medicine - Saint-Antoine Site – 4th floor
27, rue Chaligny - 75571 Paris cedex 12 - France

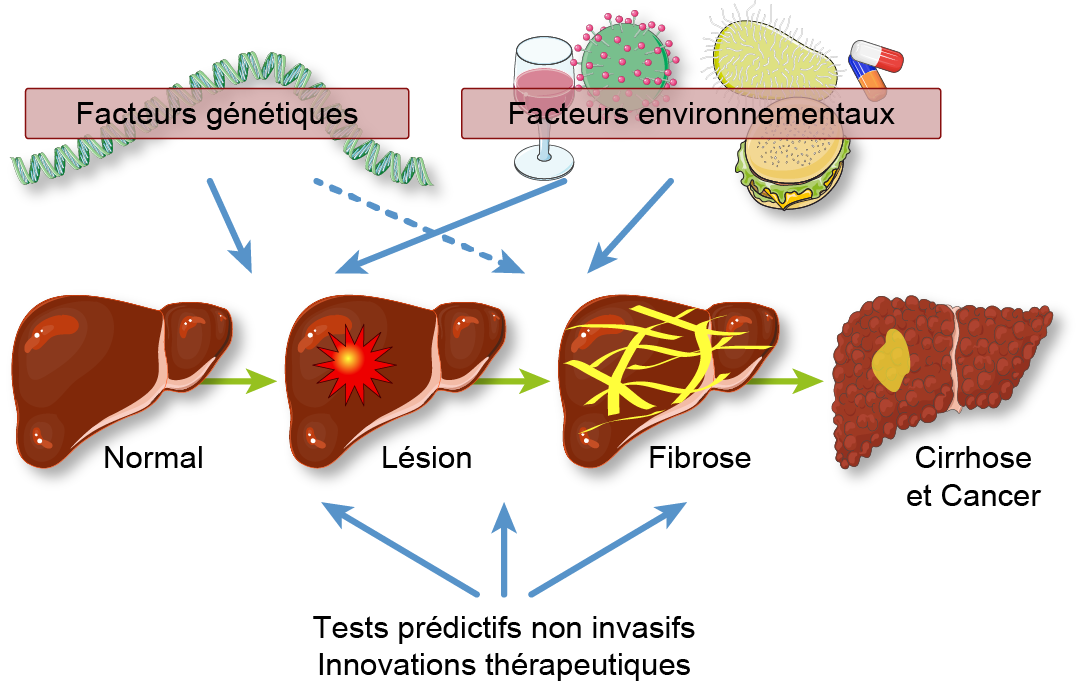
Chronic liver diseases have a major socio-economic impact. They are characterized by a continuous process of cell death and inflammation, which leads to liver fibrosis, cirrhosis and ultimately liver cancer. Our team leads a research program fully integrated with clinical hepatology, with a particular focus on biliary and metabolic diseases of the liver. We aim to uncover the cellular and molecular mechanisms underlying the pathogenesis of liver fibrosis, from its initial drivers (i.e., inherited molecular defects or cell death) to its ultimate consequences (i.e., fibrosis and liver cancer). Our research projects combine human studies, animal and in vitro models, with the major goal of translating fundamental research discoveries into therapeutic innovations. Thus, we have shown the efficacy of a new treatment combining bezafibrate and ursodeoxycholic acid in primary biliary cholangitis (N Engl J Med 2018). This research is developed in collaboration with national and international consortia, notably of rare biliary diseases and cancer.
1- ABCB4 Pathobiology
The ATP-binding Cassette transporter ABCB4 is a phospholipid translocator critical for bile generation and which deficiency causes a wide range of biliary diseases. The team has identified most of the known genetic variations of the ABCB4 gene and introduced the first functional classification of these variations. The creation of a national RADICO cohort allows studies of genotype-phenotype correlation and Next-Generation Sequencing (NGS). Research projects of the team are based on a tight interplay between theoretical and experimental approaches to investigate how sequence variants in the ABCB4 gene provide insights into how mutations impact the function of the ABCB4 protein. The team is also developing specific therapeutic approaches, including drug repositioning to correct ABCB4 deficiency. A more fundamental aspect of the project is the mechannistic understanding of the regulation of membrane expression of ABCB4, particularly through the identification of its binding partners.2- Necroinflammatory aspects of hepatobiliary diseases
2- Necroinflammatory aspects of hepatobiliary diseases
Cell death is a key factor in the progression of chronic liver diseases to fibrosis and cancer. Understanding and controlling cell death mechanims is of major importance as it could lead to improving treatments. Discovered in 2009, necroptosis or programmed necrosis refers to a biological process of cell death that shares the molecular machinery of extrinsic apoptotic pathways but whose execution resembles necrosis with cell swelling, organelle dilation, and plasma membrane rupture. It thus generates significant inflammation and has been linked to numerous diseases with an inflammatory component such as primary slerosing cholangitis (PSC) and non-alcoholic steatohepatitis (NASH). We are studying the functions of necorptosis mediators in these diseases using transgenic mouse models, human tissue banks, and cell models modified by the CRISPR/cas 9 technique. We are also testing necroptosis inhibitors in the context of liver transplantation to rehabilitate steatotic grafts using a perfusion machine that has been developed in our team. Finally, we are exploring the non-canonical functions of the necroptosis mediators in metabolism.
3- Liver fibrosis & cirrhosis
The severity of all chronic liver disease, irrespective of their etiology, is closely linked to the progression of liver fibrosis. Our team develops non-invasive methods to assess liver fibrosis and explores the mechanisms and therapeutic targets of fibrosis, using preclinical models, including of genetic cell lineage in mice. The different populations of liver myofibroblasts generating fibrosis are identified, on the basis of single-cell RNA sequencing. The role of the portal fibroblasts, which we identified is investigated with particular attention as well as the impact of their ablation on liver tissue repair in mice, using genetic recombination and gene therapy. The liver-brain and liver-gut axes in the context of liver fibrosis are also explored with a particular focus on the role of microbiota.
4- Hepatobiliary cancers
Liver cancers have a very poor prognosis. They include hepatocellular carcinomas (HCC) and cholangiocarcinomas (CCA). Most often, they occur in a chronic inflammatory environment related to alcohol consumption, viral hepatitis or NASH for HCC, and to cholangiopathies such as primary sclerosing cholangitis for CCA. Our project focuses on the role of the tumor microenvironment (TME) (fibrosis and immune infiltrate) during hepatic carcinogenesis, particularly related to hepatitis B virus (HBV) infection, and on the development of local therapies to normalize the TME and thus promote the anti-tumor immune response and increase the efficacy of immunotherapies. This work involves human 3D and murine experimental models as well as clinical studies. We are also developing non-invasive tests to predict liver cancer. Our research leads us to propose innovative strategies for the management of liver cancer, using interdisciplinary approaches.
biliary diseases / non-alcoholic steatitis-hepatitis / liver fibrosis / hepathobiliary cancer / necroptosis / ABCB4 transporter /targeted pharmacotherapy/drug repositioning/ viral hepatitis / tumor microenvironment / physical-based therapies
Certification by the Fondation pour la Recherche Médicale (FRM 2020 n°EQU202003010517): Innovations in biology for therapeutic purposes in fibrosing cholangiopathies
Articles
Vacaftor-Mediated Potentiation of ABCB4 Missense Mutations Affecting Critical Motifs of the NBDs: Repositioning Perspectives for Hepatobiliary Diseases.
Jean-Louis Delaunay , Ahmad Elbahnsi, Alix Bruneau, Claire Madry, Anne-Marie Durand-Schneider , Anne Stary , Chantal Housset , Jérémie Gautheron, Isabelle Callebaut, Tounsia Aït-Slimane.
Int J Mol Sci. (2023)
Cold plasma endoscopy applied to biliary ducts: feasibility risk assessment on human-like and porcine models for the treatment of cholangiocarcinoma.
H Decauchy, A Pavy, M Camus, L Fouassier*, T Dufour*. * co-leadership.
Journal of Physics D: Applied Physics (2022)
Protective potential of the gallbladder in primary sclerosing cholangitis.
N. Cazzagon,# E. Gonzalez-Sanchez,# H. El-Mourabit, D. Wendum, D. Rainteau, L. Humbert, C. Corpechot, O.Chazouillères, L. Arrivé,* C. Housset,* S. Lemoinne* (*Co-senior).
JHEP Rep (2022)
The necroptosis-inducing pseudokinase mixed lineage kinase domain-like regulates the adipogenic differentiation of pre-adipocytes.
J. Magusto, C. Beaupère, M.B. Afonso, M. Auclair, J.L. Delaunay, P-A. Soret, G. Courtois, T. Aït-Slimane, C. Housset, I. Jéru, B. Fève, V. Ratziu, C.M.P. Rodrigues, J. Gautheron.
iScience (2022).
Diversity of the nucleic acid forms of circulating HBV in chronically infected patients and its impact on viral cycle.
A. Schnuriger, P. Soussan, J. Sotty, P. Bablon, B. Lekbaby, J. Augustin, M. Girier-Dufournier, L. Langlois, C. Dorival, F. Carrat, S. Pol, H. Fontaine, N. Sarica, C. Neuveut, C. Housset, D. Kremdsorf.
Hepatol Int (2022).
ATP-binding cassette transporters expression in rats with cirrhosis and hepatic encephalopathy.
C. Bouzbib, H. El Mourabit, D. Wendum, E. Lasnier, S. Mouri, C. Housset, D. Thabut, N. Weiss, M. Rudler.
Clin Res Hepatol Gastroenterol (2022).
A systemic mechanism of increased transendothelial migration of leukocytes through the blood-brain barrier in hepatic encephalopathy.
A. Schaefer, M. Journaux, H. El Mourabit, S. Mouri, D. Wendum, E. Lasnier, P.O. Couraud, C. Housset, D. Thabut, M. Rudler, N. Weiss.
Clin Res Hepatol Gastroenterol (2022).
Portal fibroblasts with mesenchymal stem cell features form a reservoir of proliferative myofibroblasts in liver fibrosis.
L. Lei, A. Bruneau, H. El Mourabit, J. Guegan, T. Folseraas, S. Lemoinne, T. H. Karlsen, B. Hoareau, R. Morichon, E. Gonzalez-Sanchez, C. Goumard, V. Ratziu, P. Charbord, J. Gautheron, F. Tacke, T. Jaffredo, A. Cadoret, C. Housset.
Hepatology (2022).
Loss of thymidine phosphorylase activity disrupts adipocyte differentiation and induces insulin-resistant lipoatrophic diabetes.
J. Gautheron, L. Lima, B. Akinci, J. Zammouri, M. Auclair, S. K. Ucar, S. Ozen, C. Altay, B. E. Bax, I. Nemazanyy, V. Lenoir, C. Prip-Buus, C. Acquaviva-Bourdain, O. Lascols, B. Feve, C. Vigouroux, E. Noel, I.Jeru.
BMC Med (2022).
MRCK-Alpha and Its Effector Myosin II Regulatory Light Chain Bind ABCB4 and Regulate Its Membrane Expression.
A. Bruneau, J.L. Delaunay, A.M. Durand-Schneider, V. Vauthier, A. Ben Saad, L. Aoudjehane, H. El Mourabit, R. Morichon, T. Falguieres, J. Gautheron, C. Housset, T. Ait-Slimane.
Cells (2022).
Liver stiffness measurement by vibration-controlled transient elastography improves outcome prediction in primary biliary cholangitis.
C. Corpechot, F. Carrat, F. Gaouar, F. Chau, G. Hirschfield, A. Gulamhusein, A. J. Montano-Loza, E. Lytvyak, C. Schramm, A. Pares, I. Olivas, J. E. Eaton, K. T. Osman, G. Dalekos, N. Gatselis, F. Nevens, N. Cazzagon, A. Zago, F. P. Russo, N. Abbas, P. Trivedi, D. Thorburn, F. Saffioti, L. Barkai, D. Roccarina, V. Calvaruso, A. Fichera, A. Delamarre, E. Medina-Morales, A. Bonder, V. Patwardhan, C. Rigamonti, M. Carbone, P. Invernizzi, L. Cristoferi, A. van der Meer, R. de Veer, E. Zigmond, E. Yehezkel, A. E. Kremer, A. Deibel, J. Dumortier, T. Bruns, K. Grosse, G. P. Pageaux, A. Wetten, J. Dyson, D. Jones, O. Chazouilleres, B. Hansen, V. de Ledinghen, Global, E. R. N. R.-L. P. S. Groups.
J Hepatol (2022).
Combination of fibrates with obeticholic acid is able to normalise biochemical liver tests in patients with difficult-to-treat primary biliary cholangitis.
P. A. Soret, L. Lam, F. Carrat, L. Smets, T. Berg, M. Carbone, P. Invernizzi, V. Leroy, P. Trivedi, N. Cazzagon, C. Weiler-Normann, L. Alric, I. Rosa-Hezode, A. Heurgue, J. P. Cervoni, J. Dumortier, P. Potier, O. Roux, C. Silvain, C. Bureau, R. Anty, D. Larrey, C. Levy, A. Pares, C. Schramm, F. Nevens, O. Chazouilleres, C. Corpechot.
Aliment Pharmacol Ther (2021).
EPHX1 mutations cause a lipoatrophic diabetes syndrome due to impaired epoxide hydrolysis and increased cellular senescence.
J. Gautheron, C. Morisseau, W. K. Chung, J. Zammouri, M. Auclair, G. Baujat, E. Capel, C. Moulin, Y. Wang, J. Yang, B. D. Hammock, B. Cerame, F. Phan, B. Feve, C. Vigouroux, F. Andreelli, I. Jeru.
Elife (2021).
Zinc Finger E-Box Binding Homeobox 1 Promotes Cholangiocarcinoma Progression Through Tumor Dedifferentiation and Tumor-Stroma Paracrine Signaling.
C. Lobe, M. Vallette, A. Arbelaiz, E. Gonzalez-Sanchez, L. Izquierdo, A. Pellat, N. Guedj, C. Louis, V. Paradis, J. M. Banales, C. Coulouarn, C. Housset, J. Vaquero, L. Fouassier.
Hepatology (2021).
Low-phospholipid-associated cholelithiasis syndrome: Prevalence, clinical features, and comorbidities.
C. Dong, B. Condat, M. Picon-Coste, Y. Chretien, P. Potier, B. Noblinski, L. Arrive, M. P. Hauuy, V. Barbu, A. Maftouh, F. Gaouar, K. Ben Belkacem, C. Housset, R. Poupon, D. Zanditenas, O. Chazouilleres, C. Corpechot.
JHEP Rep (2021).
Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease.
A. Majdi, L. Aoudjehane, V. Ratziu, T. Islam, M. B. Afonso, F. Conti, T. Mestiri, M. Lagouge, F. Foufelle, F. Ballenghien, T. Ledent, M. Moldes, A. Cadoret, L. Fouassier, J.L. Delaunay, T. Ait-Slimane, G. Courtois, B. Feve, O. Scatton, C. Prip-Buus, C. M. P. Rodrigues, C. Housset, J. Gautheron.
J Hepatol (2020).
Novel defatting strategies reduce lipid accumulation in primary human culture models of liver steatosis.
L. Aoudjehane, J. Gautheron, W. Le Goff, C. Goumard, J. Gilaizeau, C. S. Nget, E. Savier, M. Atif, P. Lesnik, R. Morichon, Y. Chretien, Y. Calmus, O. Scatton, C. Housset, F. Conti.
Dis Model Mech (2020).
Cold-Atmospheric Plasma Induces Tumor Cell Death in Preclinical In Vivo and In Vitro Models of Human Cholangiocarcinoma.
J. Vaquero, F. Judee, M. Vallette, H. Decauchy, A. Arbelaiz, L. Aoudjehane, O. Scatton, E. Gonzalez-Sanchez, F. Merabtene, J. Augustin, C. Housset, T. Dufour, L. Fouassier.
Cancers (Basel) (2020).
Fungi participate in the dysbiosis of gut microbiota in patients with primary sclerosing cholangitis.
S. Lemoinne, A. Kemgang, K. Ben Belkacem, M. Straube, S. Jegou, C. Corpechot, I.B.D.N. Saint-Antoine, O. Chazouilleres, C. Housset, H. Sokol.
Gut (2020).
A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis.
C. Corpechot, O. Chazouilleres, A. Rousseau, A. Le Gruyer, F. Habersetzer, P. Mathurin, O. Goria, P. Potier, A. Minello, C. Silvain, A. Abergel, M. Debette-Gratien, D. Larrey, O. Roux, J. P. Bronowicki, J. Boursier, V. de Ledinghen, A. Heurgue-Berlot, E. Nguyen-Khac, F. Zoulim, I. Ollivier-Hourmand, J. P. Zarski, G. Nkontchou, S. Lemoinne, L. Humbert, D. Rainteau, G. Lefevre, L. de Chaisemartin, S. Chollet-Martin, F. Gaouar, F. H. Admane, T. Simon, R. Poupon.
N Engl J Med (2018).
Functional defect of variants in the adenosine triphosphate-binding sites of ABCB4 and their rescue by the cystic fibrosis transmembrane conductance regulator potentiator, ivacaftor (VX-770).
J.L. Delaunay, A. Bruneau, B. Hoffmann, A.M. Durand-Schneider, V. Barbu, E. Jacquemin, M. Maurice, C. Housset, I. Callebaut, T. Ait-Slimane.
Hepatology (2017).
Criteria for preclinical models of cholangiocarcinoma: scientific and medical relevance.
D.F. Calvisi, L. Boulter, J. Vaquero, A. Saborowski, L. Fabris, P. Rodrigues, C. Coulouarn, R. Castro, O. Segatto, C. Raggi, L. van der Laan, G. Carpino, B. Goeppert, S. Roessler, T. Kendall, M. Evert, E. Gonzalez-Sanchez, J. Valle, A. Vogel, J. Bridgewater, M. Borad, G. Gores, L. Roberts, J. Marin, J. Andersen, D. Alvaro, A. Forner, J. Banales, V. Cardinale, R. Macias, S. Vicent, X. Chen, C. Braconi, M. Verstegen, L. Fouassier.
Nat Rev Gastroenterol Hepatol. (2023).
Targeting the complexity of ERBB2 biology in gastroesophageal adenocarcinomas.
J Augustin, P Soussan, A.J. Bass.
Annals of Oncology (2022).
Alternative splicing of viral transcripts: the dark side of HBV.
D. Kremsdorf, B. Lekbaby, P. Bablon, J. Sotty, J. Augustin, A. Schnuriger, J. Pol, P. Soussan.
Gut (2021).
Lytic cell death in metabolic liver disease.
J. Gautheron, G. J. Gores, C. M. P. Rodrigues.
J Hepatol (2020).
Genetic alterations shaping tumor response to anti-EGFR therapies.
J Vaquero, A Pavy, E Gonzalez-Sanchez, M Meredith, A Arbelaiz, L Fouassier.
Drug Resistance Update (2022).
Role of Angiogenesis in the Pathogenesis of NAFLD.
L. Lei, H. Ei Mourabit, C. Housset, A. Cadoret, S. Lemoinne.
J Clin Med (2021).
In Vitro and In Vivo Models of Non-Alcoholic Fatty Liver Disease: A Critical Appraisal.
P. A. Soret, J. Magusto, C. Housset, J. Gautheron.
J Clin Med (2020).
Hepatic encephalopathy: Another brick in the wall.
N. Weiss, C. Housset, D. Thabut.
J Hepatol (2019).
New treatments/targets for primary biliary cholangitis.
C. Corpechot, R. Poupon, O. Chazouilleres.
JHEP Rep (2019).
INSERM
Kourilsky Building
34 rue Crozatier - 75012 Paris
France
Sorbonne Université Medicine
Saint-Antoine Site
27 rue Chaligny - 75012 Paris
France
