
Sorbonne Université - Faculty of medicine - Saint-Antoine Site – 7th floor
27, rue Chaligny - 75571 Paris cedex 12 - France
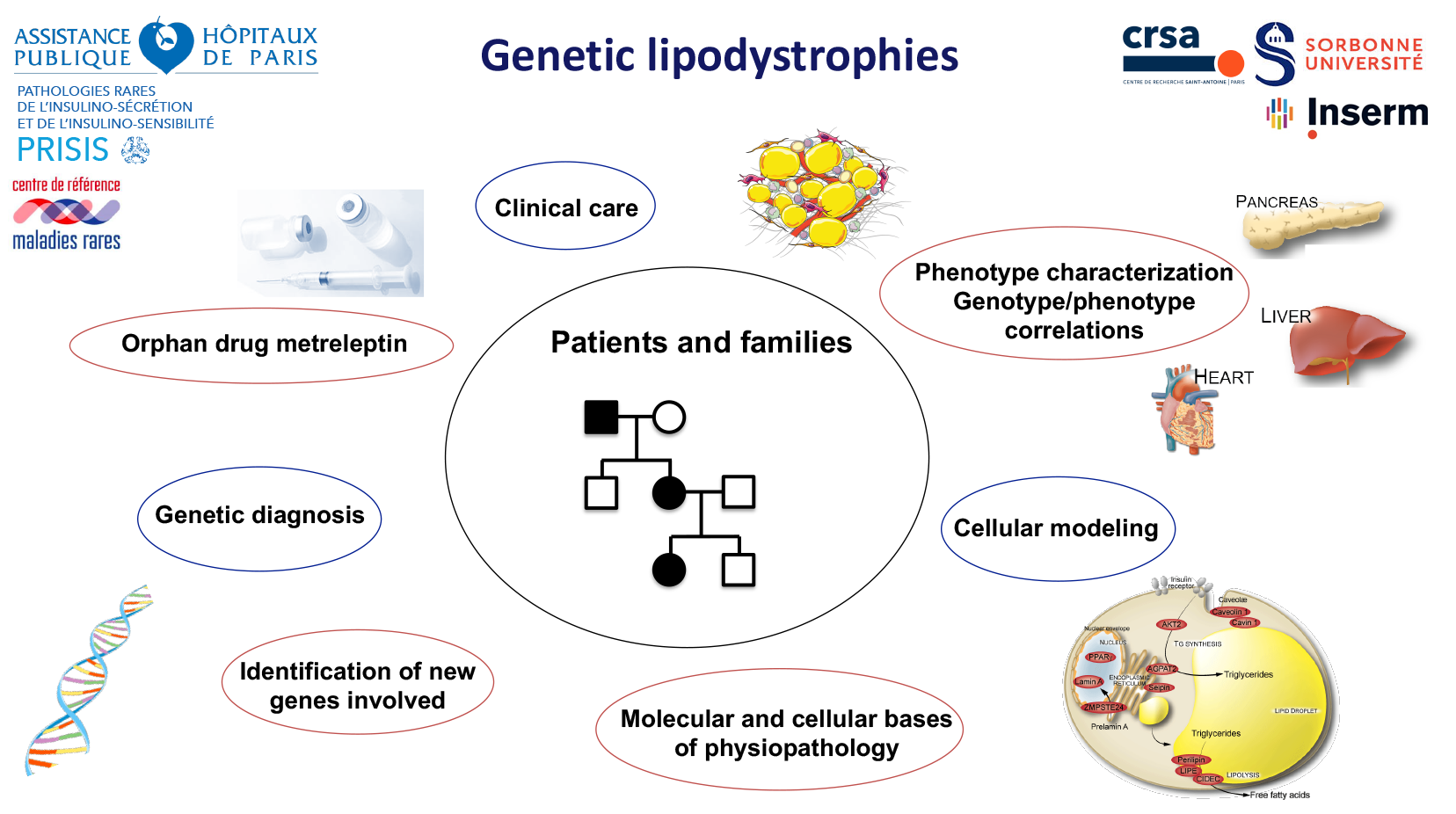
During the present contract, our translational research was focused on the pathophysiology and care of genetic and acquired lipodystrophies (LD). Our team is recognized at the national and international level for studies in this field. We investigate the different aspects of these diseases using translational approaches: search on the pathophysiological mechanisms, consequences on aging, genetic, diagnosis and prognostic factors, evaluation and improvements of patient’s care, and for therapeutic strategies. One of the major strengths of the team is our ability to develop both basic and clinical approaches through the collaborative expertise of scientists and physicians. Moreover, our team conducted many productive collaborations with teams within the Centre de Recherche Saint-Antoine (CRSA), within the IHU ICAN, and with other regional, national, and international teams to increase the quality of our research programs.
Our team has developed four different themes in the context of genetic and acquired lipodystrophies, and their systemic metabolic adaptations, aging consequences, and impact on fertility and reproductive function.

For studying these rare genetic LD, we have the unique opportunity to gather complementary expertise in genetics, cell biology, sophisticated clinical pathophysiological investigation, and care of the patients with development of several tools for educational therapy. The group has a position of national expert in these diseases, and was recognized in 2017 by the creation of the CRMR (Centre Référence Maladies Rares) PRISIS (Pathologies Rares de l’Insulino-sensibilité et de l’Insulino-sécrétion/Rare Diseases of Insulin Secretion and Insulin Sensitivity), coordinated by C. Vigouroux. This reference center was renewed in 2023 under her direction. The group is also part of the European Consortium of Lipodystrophies (ECLip) and of the European Rare Disease network Endo-ERN, Main Thematic Group “Genetic disorders of Glucose & Insulin Homeostasis”
C. Vigouroux (PUPH-HDR); C. Vatier (MCUPH) ; B. Fève (PUPH-HDR) ; F Boccara (PUPH-HDR) ; S Fellahi (PH) ; S. Janmaat - in charge of the PRISIS rare disease center; E. Capel (AI); M. Auclair (IE); K. Poussin (IE).
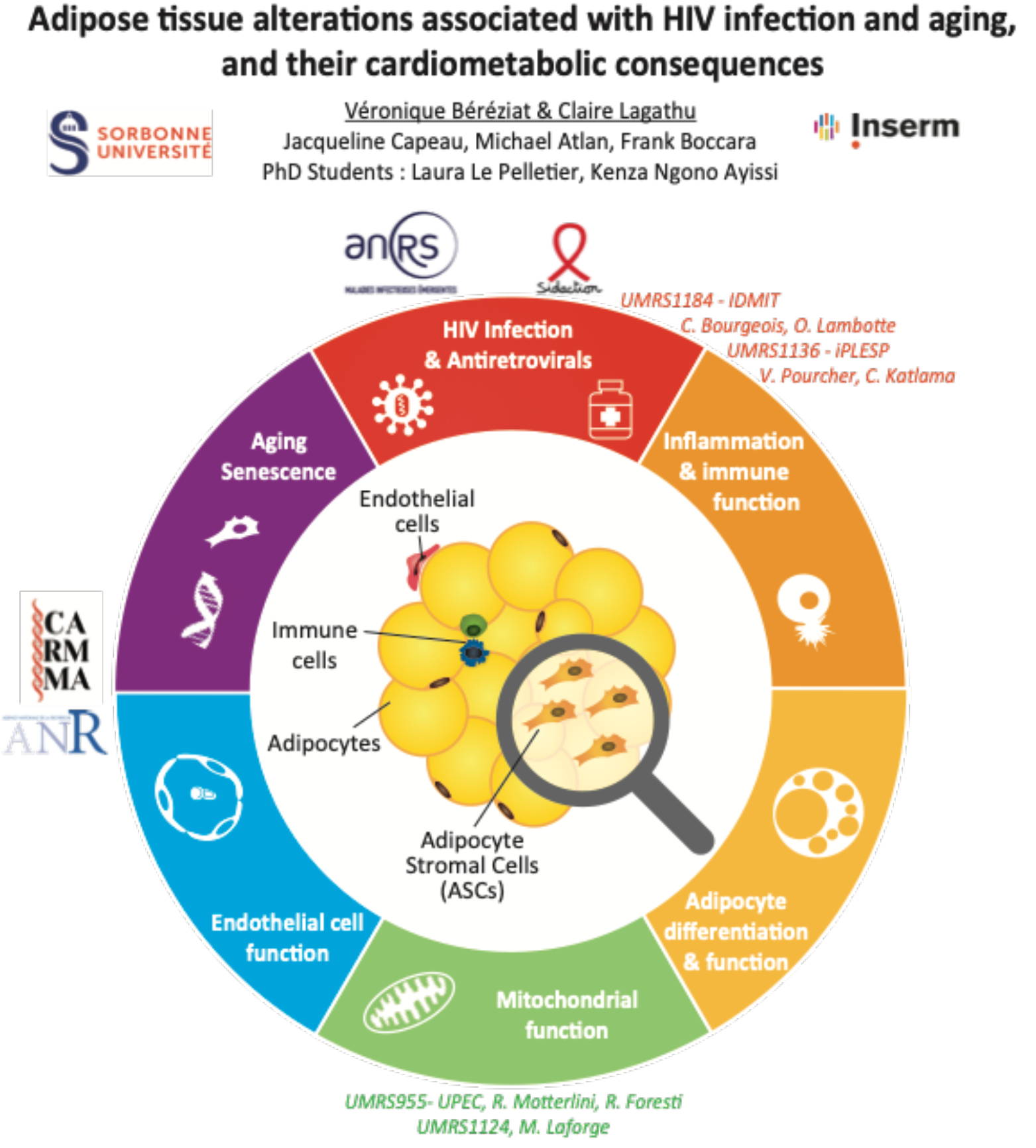
This group has studied HIV-related LD syndromes since their emergence. The phenotype of treated individuals has largely changed over the years, and is now mainly characterized by a central or generalized fat hypertrophy, and possibly by an accentuated adipose tissue aging with subsequent cardio-metabolic complications. Both in vitro approaches, in vivo preclinical studies in macaques, and clinical research on cohorts of persons living with HIV are performed to understand the pathophysiology of these complications. They could be linked either to some HIV proteins, or to specific antiretroviral treatments, or the combination of both.
C. Lagathu-Lafon (MCU-HDR); V. Béréziat (PU-HDR); J. Capeau (Prof Emeritus); F. Boccara (PUPH- HDR); M. Atlan PUPH-HDR); B. Fève (PUPH-HDR) ; S. Fellahi (PH); C. Vigouroux (PUPH-HDR) M. Auclair (IE); E. Capel (AI); L. Le Pelletier (PhD student); K. Ngono Ayissi (PhD student); T. Rajkumar (M2).
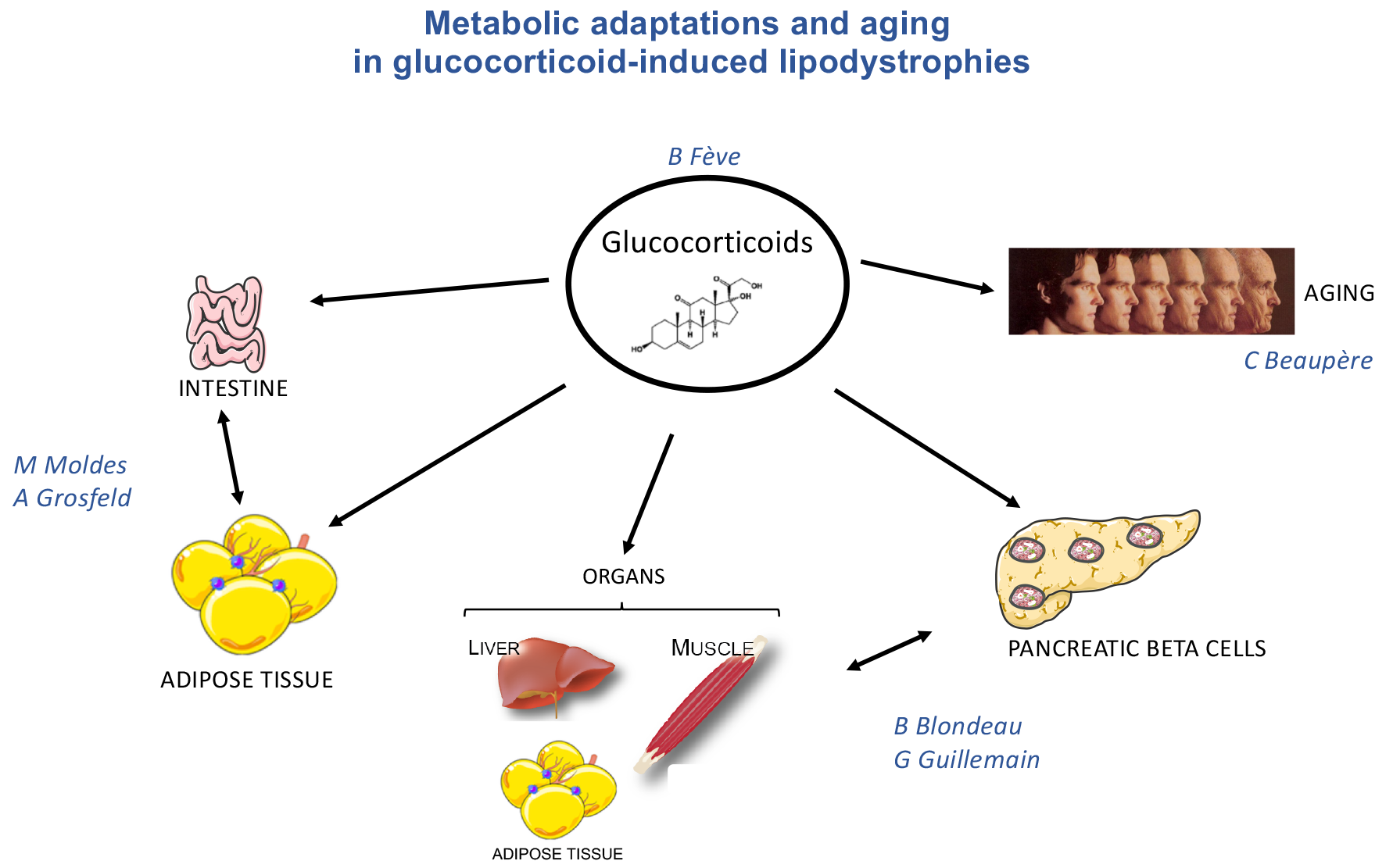
Long term endogenous or exogenous exposure to glucocorticoids is frequently associated with altered fat depot distribution, leading to severe insulin resistance, and impaired glucose and lipid homeostasis. In this field, we combine clinical studies, murine transgenic models that manipulate glucocorticoid sensitivity, and in vitro cellular models to understand the pathophysiology of this LD, and the related metabolic and endocrine adaptations, with a specific focus on adipose tissue and endocrine pancreas.
B Fève (PUPH-HDR):
Adipose tissue/gut line of research: M. Moldes (CRCN-HDR INSERM); A. Grosfeld (MCU); A. Vali (PhD student) ; A. Loubaresse (PhD student); P. Moreau (M2), C Zhu (AI contract)
Adipose tissue/pancreas line of research: B. Blondeau (PU-HDR) ; G. Guillemain (CRCN-HDR INSERM) ; C. Beaupère (post-doctoral fellow) ; A. Liboz (PhD student) ; E. Rousseau (M2)
Glucocorticoids and aging line of research: C. Beaupère (CRCN)
Glucocorticoids, energy homeostasis and thermogenesis line of research: B. Antoine (CRCN CNRS) ; N. Roblot (TN) ; K. Poussin (IE) ; M. Auclair (IE).
This axis gathers the excellent skills of all the senior investigators, in strong interaction with members of the other axes. There is a sum of evidence showing that genetic or acquired LD have consequences on fertility, with polycystic ovaries and anovulation in women, and sperm defects in men. Both in vitro and in vivo models, and clinical investigations on large cohorts are performed to decipher the mechanisms underlying the relationships between adipose tissue disorders and deregulations of reproductive functions.
N Di Clemente (DR-HDR INSERM) ; R Levy (PUPH-HDR) ; C Dupont (MCUPH) ; C Racine (MCU HDR) ; E. Mathieu D'Argent (PH) ; N. Sermonade (PH) ; L Bardet (PH) ; A. Ly (assistant specialist) ; C. Panissard (Assicatnt specialist); G Bachelot (AHU) ; JY. Picard (volunteer DR INSERM) ; N. Roblot (TN); K. Poussin (IE) ; I Berthaut (IE) ; C Goblet (M2) ;
Dunnigan lipodystrophy syndrome: French National Diagnosis and Care Protocol (PNDS; Protocole National de Diagnostic et de Soins), Mosbah H, Donadille B, Vatier C, Janmaat S, Atlan M, Badens C, Barat P, Beliard S, Beltrand J, Ben Yaou R, Bismuth E, Boccara F, Cariou B, Chaouat M, Charriot G, Christin-Maitre S, De Kerdanet M, Delemer B, Disse E, Dubois N, Eymard B, Feve B, Lascols O, Mathurin P, Nobecourt E, Poujol-Robert A, Prevost G, Richard P, Sellam J, Tauveron I, Treboz D, Verges B, Vermot-Desroches V, Wahbi K, Jeru I, Vantyghem MC, Vigouroux C Orphanet Journal of Rare Diseases 2022 WOS:000783817200001
Proceedings of the annual meeting of the European Consortium of Lipodystrophies (ECLip) Cambridge, UK, 7-8 April 2022, Mosbah H, Akinci B, Araujo-Vilar D, Tudela JC, Ceccarini G, Collas P, Farooqi IS, Fernandez-Pombo A, Jeru I, Karpe F, Krause K, Maffei M, Miehle K, Oral E, de Tudela NP, Prieur X, Rochford J, Sanders R, Santini F, Savage DB, von Schnurbein J, Semple R, Stears A, Sorkina E, Vantyghem MC, Vatier C, Vidal-Puig A, Vigouroux C, Wabitsch M, Annales d’Endocrinologie 2022 WOS:000897862500013
Therapeutic indications and metabolic effects of metreleptin in patients with lipodystrophy syndromes: Real-life experience from a national reference network, Mosbah H, Vantyghem MC, Nobecourt E, Andreelli F, Archambeaud F, Bismuth E, Briet C, Cartigny M, Chevalier B, Donadille B, Daguenel A, Fichet M, Gautier JF, Janmaat S, Jeru I, Legagneur C, Leguier L, Maitre J, Mongeois E, Poitou C, Renard E, Reznik Y, Spiteri A, Travert F, Verges B, Zammouri J, Vigouroux C, Vatier C, Diabetes Obesity & Metabolism 2022 WOS:000793674100001
Role of insulin resistance on fertility - Focus on polycystic ovary syndrome, Vatier C, Christin-Maitre S, Vigouroux C, Annales D Endocrinologie 2022 WOS:000808105100013
Molecular and Cellular Bases of Lipodystrophy Syndromes, Zammouri J, Vatier C, Capel E, Auclair M, Storey-London C, Bismuth E, Mosbah H, Donadille B, Janmaat S, Feve B, Jeru I, Vigouroux C, Frontiers in Endocrinology 2022 WOS:000750603600001
Loss of thymidine phosphorylase activity disrupts adipocyte differentiation and induces insulin-resistant lipoatrophic diabetes, Gautheron J, Lima L, Akinci B, Zammouri J, Auclair M, Ucar SK, Ozen S, Altay C, Bax BE, Nemazanyy I, Lenoir V, Prip-Buus C, Acquaviva-Bourdain C, Lascols O, Feve B, Vigouroux C, Noel E, Jeru I, Bmc Medicine 2022 WOS:000773402400001
RIPK3 dampens mitochondrial bioenergetics and lipid droplet dynamics in metabolic liver disease, Afonso MB, Islam T, Magusto J, Amorim R, Lenoir V, Simoes RF, Teixeira J, Silva LC, Wendum D, Jeru I, Vigouroux C, Castro RE, Oliveira PJ, Prip-Buus C, Ratziu V, Gautheron J, Rodrigues CMP, Hepatology 2022 WOS:000866148100001
EPHX1 mutations cause a lipoatrophic diabetes syndrome due to impaired epoxide hydrolysis and increased cellular senescence, Gautheron J, Morisseau C, Chung WK, Zammouri J, Auclair M, Baujat G, Capel E, Moulin C, Wang YX, Yang J, Hammock BD, Cerame B, Phan F, Feve B, Vigouroux C, Andreelli F, Jeru I Elife 2021 WOS:000683009800001
A recurrent familial partial lipodystrophy due to a monoallelic or biallelic LMNA founder variant highlights the multifaceted cardiac manifestations of metabolic laminopathies, Treiber G, Furmaniuk AF, Guilleux A, Medjane S, Bonfanti O, Schneebeli S, Bernard C, Le-Moullec N, Bakiri F, Pholsena M, Rollot O, Vatier C, Jarlet E, Jeru I, Lascols O, Darcel F, Domun B, Venault A, Venault S, Jacquemont ML, Doray B, Maiza JC, Cogne M, Vigouroux C, Nobecourt E, European Journal of Endocrinology 2021 WOS:000691799900005
Biallelic CAV1 null variants induce congenital generalized lipodystrophy with achalasia, Karhan AN, Zammouri J, Auclair M, Capel E, Apaydin FD, Ates F, Verpont MC, Magre J, Feve B, Lascols O, Usta Y, Jeru I, Vigouroux C European Journal of Endocrinology, 2021 WOS:000750678000008
Two Decades after Mandibuloacral Dysplasia Discovery: Additional Cases and Comprehensive View of Disease Characteristics, Jeru I, Nabil A, El-Makkawy G, Lascols O, Vigouroux C, Abdalla E Genes 2021 WOS:000712667500001
Factors Associated With Being Overweight and Obesity in People Living With Human Immunodeficiency Virus on Antiretroviral Therapy: Socioclinical, Inflammation, and Metabolic Markers, Goupil de Bouille J, Vigouroux C, Plessis L, Ghislain M, Teglas JP, Boufassa F, Goujard C, Vignes D, Bouchaud O, Salmon D, Meyer L, Abgrall S Journal of Infectious Diseases, 2021 WOS:000728427500015
Laminopathies' Treatments Systematic Review: A Contribution Towards a 'Treatabolome', Atalaia A, Ben Yaou R, Wahbi K, De Sandre-Giovannoli A, Vigouroux C, Bonne G Journal of Neuromuscular Diseases 2021 WOS:000684017400006
Autoimmune hypoglycemia expands the biological spectrum of HHV8(+) multicentric Castleman disease, Arnautou P, Auclair M, Fellahi S, Bouche C, Fieschi C, Barrak E, Queyrel-Moranne V, Chaillous L, Blin N, Malphettes M, Fadlallah J, Bertinchamp R, Gerard L, Bengoufa D, Galicier L, Oksenhendler E, Vigouroux C, Boutboul D, Blood Advances, 2021 WOS:000640206000008
LIPE-related lipodystrophic syndrome: clinical features and disease modeling using adipose stem cells. Sollier C, Capel E, Aguilhon C, Smirnov V, Auclair M, Douillard C, Ladsous M, Defoort-Dhellemmes S, Gorwood J, Braud L, Motterlini R, Vatier C, Lascols O, Renard E, Vigouroux C, Jéru I. Eur J Endocrinol. 2021. PMID: 33112291.
The Multifaceted Role of Epoxide Hydrolases in Human Health and Disease. Gautheron J, Jéru I. Int J Mol Sci. 2020. PMID: 33374956. Review
Lipodystrophic syndromes: From diagnosis to treatment. Sollier C, Vatier C, Capel E, Lascols O, Auclair M, Janmaat S, Fève B, Jéru I, Vigouroux C. Ann Endocrinol. 2020. PMID: 31982105.
Overlapping phenotypes between SHORT and Noonan syndromes in patients with PTPN11 pathogenic variants. Ranza E, Guimier A, Verloes A, Capri Y, Marques C, Auclair M, Mathieu-Dramard M, Morin G, Thevenon J, Faivre L, Thauvin-Robinet C, Innes AM, Dyment DA, Vigouroux C, Amiel J. Clin Genet. 2020. PMID: 32233106.
Congenital Generalized Lipoatrophy (Berardinelli-Seip Syndrome) Type 1: Description of Novel AGPAT2 Homozygous Variants Showing the Highly Heterogeneous Presentation of the Disease. Ceccarini G, Magno S, Pelosini C, Ferrari F, Sessa MR, Scabia G, Maffei M, Jéru I, Lascols O, Vigouroux C, Santini F. Front Endocrinol (Lausanne). 2020. PMID: 32117065.
Progerin Expression Induces Inflammation, Oxidative Stress and Senescence in Human Coronary Endothelial Cells. Bidault G, Garcia M, Capeau J, Morichon R, Vigouroux C, Béréziat V. Cells. 2020. PMID: 32408587.
Looking at New Unexpected Disease Targets in LMNA-Linked Lipodystrophies in the Light of Complex Cardiovascular Phenotypes: Implications for Clinical Practice. Mosbah H, Vatier C, Boccara F, Jéru I, Lascols O, Vantyghem MC, Fève B, Donadille B, Sarrazin E, Benabbou S, Inamo J, Ederhy S, Cohen A, Neraud B, Richard P, Picard F, Christin-Maitre S, Redheuil A, Wahbi K, Vigouroux C. Cells. 2020. PMID: 32245113.
Diagnostic Challenge in PLIN1-Associated Familial Partial Lipodystrophy. Jéru I, Vantyghem MC, Bismuth E, Cervera P, Barraud S; PLIN1-Study Group, Auclair M, Vatier C, Lascols O, Savage DB, Vigouroux C. J Clin Endocrinol Metab. 2019. PMID: 31504636.
Adherence with metreleptin therapy and health self-perception in patients with lipodystrophic syndromes. Vatier C, Kalbasi D, Vantyghem MC, Lascols O, Jéru I, Daguenel A, Gautier JF, Buyse M, Vigouroux C. Orphanet J Rare Dis. 2019. PMID: 31300002.
MFN2-associated lipomatosis: Clinical spectrum and impact on adipose tissue. Capel E, Vatier C, Cervera P, Stojkovic T, Disse E, Cottereau AS, Auclair M, Verpont MC, Mosbah H, Gourdy P, Barraud S, Miquel A, Züchner S, Bonnefond A, Froguel P, Christin-Maitre S, Delemer B, Fève B, Laville M, Robert J, Tenenbaum F, Lascols O, Vigouroux C, Jéru I. J Clin Lipidol. 2018. PMID: 30158064.
The lipodystrophic hotspot lamin A p.R482W mutation deregulates the mesodermal inducer T/Brachyury and early vascular differentiation gene networks. Briand N, Guénantin AC, Jeziorowska D, Shah A, Mantecon M, Capel E, Garcia M, Oldenburg A, Paulsen J, Hulot JS, Vigouroux C, Collas P. Hum Mol Genet. 2018. PMID: 29438482.
Inhibition of Adipose Tissue Beiging by HIV Integrase Inhibitors, Dolutegravir and Bictegravir, Is Associated with Adipocyte Hypertrophy, Hypoxia, Elevated Fibrosis, and Insulin Resistance in Simian Adipose Tissue and Human Adipocytes, Ayissi KN, Gorwood J, Le Pelletier L, Bourgeois C, Beaupere C, Auclair M, Foresti R, Motterlini R, Atlan M, Barrail-Tran A, Le Grand R, Desjardins D, Fève B, Lambotte O, Capeau J, Béréziat V*, Lagathu C* Cells 2022 WOS:000808762800001 * equal contribution
Prolonged Antiretroviral Treatment Induces Adipose Tissue Remodelling Associated with Mild Inflammation in SIV-Infected Macaques. Mausoléo A, Olivo A, Desjardins D, Sáez-Cirión A, Barrail-Tran A, Avettand-Fenoel V, Noël N, Lagathu C, Béréziat V, Le Grand R, Lambotte O, Bourgeois C. Cells. 2022 PMID: 36231066
Comparison of HIV-Infected and Noninfected Patients Undergoing Bariatric Surgery: The ObeVIH Study, Pourcher V, Capeau J, Dudoit Y, Boccara F, Soulie C, Ndoadoumgue AL, Charlotte F, Fellahi S, Bastard JP, Béreéziat V, Lagathu C, Marcelin AG, Peytavin G, Boutron-Ruault MC, Tubbax C, D'Auerstaedt AD, Valantin MA, Schneider L, Costagliola D, Katlama C, Assoumou L, Pourcher G, Jaids-Journal of Acquired Immune Deficiency Syndromes 2022 WOS:000811052700017
Contribution of Adipose Tissue to the Chronic Immune Activation and Inflammation Associated With HIV Infection and Its Treatment, Bourgeois C, Gorwood J, Olivo A, Le Pelletier L, Capeau J, Lambotte O, Béréziat V*, Lagathu C* Front Endoc, 2021, WOS:000668927500001 Review .* equal contribution
Metformin alleviates stress-induced cellular senescence of aging human adipose stromal cells and the ensuing adipocyte dysfunction Le Pelletier L, Mantecon M, Gorwood J, Auclair M, Foresti R, Motterlini R, Laforge M, Atlan M, Fève B, Capeau J, Lagathu C*, Béréziat V*. eLife. 2021. PMID: 34544550* equal contribution
Prevalence of Silent Atherosclerosis and Other Comorbidities in an Outpatient Cohort of Adults Living with HIV: Associations with HIV Parameters and Biomarkers. Ghosn J, Abdoul H, Fellahi S, Merlet A, Salmon D, Morini JP, Deleuze J, Blacher J, Capeau J, Bastard JP, Viard JP. AIDS Res Hum Retroviruses. 2021. PMID: 33076677
Altered subcutaneous adipose tissue parameters after switching ART-controlled HIV+ patients to raltegravir/maraviroc. Bastard JP, Pelloux V, Alili R, Fellahi S, Aron-Wisnewsky J, Capel E, Fève B, Assoumou L, Prifti E, Katlama C, Clément K, Capeau J. AIDS. 2021. PMID: 33831906
Recent data on adipose tissue, insulin resistance, diabetes and dyslipidaemia in antiretroviral therapy controlled HIV-infected persons. Capeau J, Lagathu C, Béréziat V, Fève B. Curr Opin HIV AIDS. 2021 PMID: 33783403
HIV and antiretroviral therapy-related fat alterations. Koethe JR, Lagathu C, Lake JE, Domingo P, Calmy A, Falutz J, Brown TT, Capeau J. Nat Rev Dis Primers. 2020. PMID: 32555389 Review.
The Integrase Inhibitors Dolutegravir and Raltegravir Exert Proadipogenic and Profibrotic Effects and Induce Insulin Resistance in Human/Simian Adipose Tissue and Human Adipocytes. Gorwood J, Bourgeois C, Pourcher V, Pourcher G, Charlotte F, Mantecon M, Rose C, Morichon R, Atlan M, Le Grand R, Desjardins D, Katlama C, Fève B, Lambotte O, Capeau J, *Béréziat V, *Lagathu C. Clin Infect Dis. 2020. PMID: 32166319. * equal contribution
Progerin Expression Induces Inflammation, Oxidative Stress and Senescence in Human Coronary Endothelial Cells. Bidault G, Garcia M, Capeau J, Morichon R, Vigouroux C, Béréziat V. Cells. 2020. PMID: 32408587
Fat gain differs by sex and hormonal status in persons living with suppressed HIV switched to raltegravir/etravirine. Assoumou L, Racine C, Fellahi S, Lamaziere A, Farabos D, Beniguel L, Bastard JP, Fève B, Gibowski S, Katlama C, Costagliola D, Capeau J. AIDS. 2020. PMID: 32773470
SIV Infection and the HIV Proteins Tat and Nef Induce Senescence in Adipose Tissue and Human Adipose Stem Cells, Resulting in Adipocyte Dysfunction. Gorwood J, Ejlalmanesh T, Bourgeois C, Mantecon M, Rose C, Atlan M, Desjardins D, Le Grand R, Fève B, Lambotte O, Capeau J, *Béréziat V, *Lagathu C. Cells. 2020 PMID: 32244726 * equal contribution
Specific Biological Features of Adipose Tissue, and Their Impact on HIV Persistence. Bourgeois C, Gorwood J, Barrail-Tran A, Lagathu C, Capeau J, Desjardins D, Le Grand R, Damouche A, Béréziat V, Lambotte O. Front Microbiol. 2019. eCollection 2019.PMID: 31921023 Review.
Impact of HIV/simian immunodeficiency virus infection and viral proteins on adipose tissue fibrosis and adipogenesis. Gorwood J, Bourgeois C, Mantecon M, Atlan M, Pourcher V, Pourcher G, Le Grand R, Desjardins D, Fève B, Lambotte O, Capeau J, *Béréziat V, *Lagathu C. AIDS. 2019 PMID: 30946149 * equal contribution
Metabolic complications affecting adipose tissue, lipid and glucose metabolism associated with HIV antiretroviral treatment. Lagathu C, Béréziat V, Gorwood J, Fellahi S, Bastard JP, Vigouroux C, Boccara F, Capeau J. Expert Opin Drug Saf. 2019. PMID: 31304808. Review.
Systemic Dysfunction of Osteoblast Differentiation in Adipose-Derived Stem Cells from Patients with Multiple Myeloma. Béréziat V*, Mazurier C*, Auclair M, Ferrand N, Jolly S, Marie T, Kobari L, Toillon I, Delhommeau F, Fève B, Larsen AK, Sabbah M, Garderet L. Cells. 2019 PMID: 31083455 *equal contribution
Breast-Associated Adipocytes Secretome Induce Fatty Acid Uptake and Invasiveness in Breast Cancer Cells via CD36 Independently of Body Mass Index, Menopausal Status and Mammary Density. Zaoui M, Morel M, Ferrand N, Fellahi S, Bastard JP, Lamazière A, Larsen AK, Béréziat V, Atlan M, Sabbah M. Cancers (Basel). 2019. PMID: 31847105
Lipodystrophic syndromes due to LMNA mutations: recent developments on biomolecular aspects, pathophysiological hypotheses and therapeutic perspectives. Vigouroux C, Guénantin AC, Vatier C, Capel E, Le Dour C, Afonso P, Bidault G, Béréziat V, Lascols O, Capeau J, Briand N, Jéru I. Nucleus. 2018. PMID: 29578370
Interleukins in adipose tissue: Keeping the balance, Antuna-Puente B, Fellahi S, McAvoy C, Feve B, Bastard JP. Molecular and Cellular Endocrinology 2022 WOS:000793240000001
The resolvin D2-GPR18 axis is expressed in human coronary atherosclerosis and transduces atheroprotection in apolipoprotein E deficient mice, Bardin M, Pawelzik SC, Lagrange J, Mahdi A, Arnardottir H, Feve B, Regnault V, Lacolley P, Michel JB, Mercier N, Back M, Biochemical Pharmacology 2022 WOS:000806289700007
The ERICH3 rs11580409 polymorphism is associated with 6-month antidepressant response in depressed patients. Chappell K, Colle R, Tayeb AKA, Bouligand J, El-Asmar K, Deflesselle E, Feve B, Becquemont L, Corruble E, Verstuyft C, Progress in Neuro-Psychopharmacology & Biological Psychiatry 2022, WOS:000895855900010
When therapeutic drugs lead to diabetes, Feve B, Scheen AJ, Diabetologia 2022 WOS:000764623800002
Increased atherosclerotic plaque in AOC3 knock-out in ApoE(-/-) mice and characterization of AOC3 in atherosclerotic human coronary arteries, Filip A, Taleb S, Bascetin R, Jahangiri M, Bardin M, Lerognon C, Feve B, Lacolley P, Jalkanen S, Mercier N, Frontiers in Cardiovascular Medicine 2022 WOS:000861187400001
Circulating cytokines present in multiple myeloma patients inhibit the osteoblastic differentiation of adipose stem cells, Kobari L, Auclair M, Piau O, Ferrand N, Zaoui M, Delhommeau F, Feve B, Sabbah M, Garderet L Leukemia 2022 WOS:000698532100001
The GG genotype of the serotonin 4 receptor genetic polymorphism, rs1345697, is associated with lower remission rates after antidepressant treatment: Findings from the METADAP cohort, Poinsignon V, Colle R, El Asmar K, Mendez-David I, David DJ, Tayeb AKA, Chappell K, Gressier F, Herrero H, Feve B, Becquemont L, Corruble E, Verstuyft C, Journal of Affective Disorders 2022, WOS:000744027000013
Blood microbiota and metabolomic signature of major depression before and after antidepressant treatment: a prospective case-control study, Ciocan D, Cassard AM, Becquemont L, Verstuyft C, Voican CS, El Asmar K, Colle R, David D, Trabado S, Feve B, Chanson P, Perlemuter G, Corruble E, Journal of Psychiatry & Neuroscience 2021 WOS:000743686000005
Diabetes Increases Severe COVID-19 Outcomes Primarily in Younger Adults, Diedisheim M, Dancoisne E, Gautier JF, Larger E, Cosson E, Feve B, Chanson P, Czernichow S, Tatulashvili S, Raffin-Sanson ML, Sallah K, Bourgeon M, Ajzenberg C, Hartemann A, Daniel C, Moreau T, Roussel R, Potier L, Journal of Clinical Endocrinology & Metabolism 2021 WOS:000692625700030
Deficiency of the RNA-binding protein Cth2 extends yeast replicative lifespan by alleviating its repressive effects on mitochondrial function Patnaik PK, Beaupere C, Barlit H, Romero AM, Tsuchiya M, Muir M, Martinez-Pastor MT, Puig S, Kaeberlein M, Labunskyy VM, Cell reports 2022 WOS:000842374300002
Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. Beaupere C, Liboz A, Fève B, Blondeau B, Guillemain G. Int J Mol Sci. 2021. PMID: 33435513 Review
Pharmacological modulation of RORα controls fat browning, adaptive thermogenesis, and body weight in mice. Auclair M, Roblot N, Capel E, Fève B, Antoine B. Am J Physiol Endocrinol Metab. 2021. PMID: 33252251
Sex disparities in COVID-19 outcomes of inpatients with diabetes: insights from the CORONADO study. Tramunt B, Smati S, Coudol S, Wargny M, Pichelin M, Guyomarch B, Al-Salameh A, Amadou C, Barraud S, Bigot É, Bordier L, Borot S, Bourgeon M, Bourron O, Charriere S, Chevalier N, Cosson E, Fève B, Flaus-Furmaniuk A, Fontaine P, Galioot A, Gonfroy-Leymarie C, Guerci B, Lablanche S, Lalau JD, Larger E, Lasbleiz A, Laviolle B, Marre M, Munch M, Potier L, Prévost G, Renard E, Reznik Y, Seret-Begue D, Sibilia P, Thuillier P, Vergès B, Gautier JF, Hadjadj S, Cariou B, Mauvais-Jarvis F, Gourdy P. Eur J Endocrinol. 2021. PMID: 34085949
Changes in circulating miRNA19a-3p precede insulin resistance programmed by intra-uterine growth retardation in mice. Saget S, Cong R, Decourtye L, Endale ML, Martinerie L, Girardet C, Perret C, Clemessy M, Leneuve P, Dinard L, Mohand Oumoussa B, Farabos D, Lamazière A, Lombès M, Moldes M, Fève B, Tregouet D, Le Bouc Y, Kappeler L. Mol Metab. 2020 PMID: 32956848
Glucocorticoids impair HDL-mediated cholesterol efflux besides increased HDL cholesterol concentration: a proof of concept. Bouillet B, Gautier T, Denimal D, Samson M, Masson D, Pais de Barros JP, Maquart G, Xolin M, Grosfeld A, Dalle H, Vergès B, Moldes M, Fève B. Eur J Endocrinol. 2020. PMID: 32570209
Exposure to Glucocorticoids in the First Part of Fetal Life is Associated with Insulin Secretory Defect in Adult Humans. Riveline JP, Baz B, Nguewa JL, Vidal-Trecan T, Ibrahim F, Boudou P, Vicaut E, Brac de la Perrière A, Fetita S, Bréant B, Blondeau B, Tardy-Guidollet V, Morel Y, Gautier JF. J Clin Endocrinol Metab. 2020 PMID: 31665349
Insulin activates hepatic Wnt/β-catenin signaling through stearoyl-CoA desaturase 1 and Porcupine. Cabrae R, Dubuquoy C, Caüzac M, Morzyglod L, Guilmeau S, Noblet B, Fève B, Postic C, Burnol AF, Moldes M. Sci Rep. 2020 PMID: 32198362
Slug, a Cancer-Related Transcription Factor, is Involved in Vascular Smooth Muscle Cell Transdifferentiation Induced by Platelet-Derived Growth Factor-BB During Atherosclerosis. Ledard N, Liboz A, Blondeau B, Babiak M, Moulin C, Vallin B, Guillas I, Mateo V, Jumeau C, Blirando K, Meilhac O, Limon I, Glorian M. J Am Heart Assoc. 2020. PMID: 31959031
Fructose malabsorption induces cholecystokinin expression in the ileum and cecum by changing microbiota composition and metabolism. Zhang X, Grosfeld A, Williams E, Vasiliauskas D, Barretto S, Smith L, Mariadassou M, Philippe C, Devime F, Melchior C, Gourcerol G, Dourmap N, Lapaque N, Larraufie P, Blottière HM, Herberden C, Gerard P, Rehfeld JF, Ferraris RP, Fritton JC, Ellero-Simatos S, Douard V. FASEB J. 2019. PMID: 30939042
Adipocyte Glucocorticoid Receptor Deficiency Promotes Adipose Tissue Expandability and Improves the Metabolic Profile Under Corticosterone Exposure. Dalle H, Garcia M, Antoine B, Boehm V, Do TTH, Buyse M, Ledent T, Lamazière A, Magnan C, Postic C, Denis RG, Luquet S, Fève B, Moldes M. Diabetes. 2019 PMID: 30455377
Adaptive β-Cell Neogenesis in the Adult Mouse in Response to Glucocorticoid-Induced Insulin Resistance. Courty E, Besseiche A, Do TTH, Liboz A, Aguid FM, Quilichini E, Buscato M, Gourdy P, Gautier JF, Riveline JP, Haumaitre C, Buyse M, Fève B, Guillemain G, Blondeau B. Diabetes. 2019 PMID: 30327384
Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E. Science. 2018. PMID: 29519916
Antenatal antipsychotic exposure induces multigenerational and gender-specific programming of adiposity and glucose tolerance in adult mouse offspring. Courty E, Gobalakichenane P, Garcia M, Muscat A, Kazakian C, Ledent T, Moldes M, Blondeau B, Mitanchez D, Buyse M, Fève B. Diabetes Metab. 2018. PMID: 28729164
The anti-Mullerian hormone prodomain is displaced from the hormone/prodomain complex upon bivalent binding to the hormone receptor, Cate RL, di Clemente N, Racine C, Groome NP, Pepinsky RB, Whitty A, Journal of Biological Chemistry 2022, WOS:000765984600009
Anti-Mullerian Hormone and Polycystic Ovary Syndrome in Women and Its Male Equivalent, di Clemente N, Racine C, Rey RA, Biomedicines 2022 WOS:000872195200001
Combining metabolomics and machine learning models as a tool to distinguish non-classic 21-hydroxylase deficiency from polycystic ovary syndrome without adrenocorticotropic hormone testing, Bachelot G, Bachelot A, Bonnier M, Salem JE, Farabos D, Trabado S, Dupont C, Kamenicky P, Houang M, Fiet J, Le Bouc Y, Young J, Lamaziere A, Human Reproduction 2022, WOS:000891997700001
Impact of Bariatric Surgery-Induced Weight Loss on Ovarian Reserve in Women with Obesity: A Systematic Review, Dupont C, Didon S, Ciangura C, Selleret L, Bachelot A, Levy R, Sermondade N, Bariatric Surgical Practice and Patient Care 2022 WOS:000682038900001
Association between metabolic disorders and seminal plasma miRNA levels: a pilot study Saget S, Kappeler L, Grandjean V, Leneuve P, Berthaut I, Faure C, Czernichow S, Racine C, Levy R, Dupont C, Basic and Clinical Andrology 2022, WOS:000806783900001
Persistent Mullerian duct syndrome associated with genetic defects in the regulatory subunit of myosin phosphatase, Picard JY, Morin G, Devouassoux-Shisheboran M, Van der Smagt J, Klosowski S, Pienkowski C, Pierre-Renoult P, Masson C, Bole C, Josso N, Human Reproduction 2022 WOS:000878757300001
The netrin-1 receptor UNC5C contributes to the homeostasis of undifferentiated spermatogonia in adult mice, Barroca V, Racine C, Pays L, Fouchet P, Coureuil M, Allemand I, Stem Cell Research 2022 WOS:000792694400002
Anti-Mullerian Hormone in Female Reproduction, di Clemente N, Racine C, Pierre A, Taieb J, Endocrine Reviews 2021 WOS:000728161000003
DYRK1A Overexpression in Mice Downregulates the Gonadotropic Axis and Disturbs Early Stages of Spermatogenesis, Dard R, Moreau M, Parizot E, Ghieh F, Brehier L, Kassis N, Serazin V, Lamaziere A, Racine C, di Clemente N, Vialard F, Janel N, Genes 2021 WOS:000724887600001
Proof of concept and development of a couple-based machine learning model to stratify infertile patients with idiopathic infertility, Bachelot G, Levy R, Bachelot A, Faure C, Czernichow S, Dupont C, Lamaziere A, Scientific Reports 2021, WOS:000730779300074
Fertility preservation in young men with Klinefelter syndrome: A systematic review, Ly A, Sermondade N, Brioude F, Berthaut I, Bachelot A, Hamid RH, El Khattabi L, Prades M, Levy R, Dupont C, Journal of Gynecology Obstetrics and Human Reproduction 2021 WOS:000703904000029
New Anti-Müllerian Hormone Target Genes Involved in Granulosa Cell Survival in Women With Polycystic Ovary Syndrome. Racine C, Genêt C, Bourgneuf C, Dupont C, Plisson-Petit F, Sarry J, Hennequet-Antier C, Vigouroux C, Mathieu d'Argent E, Pierre A, Monniaux D, Fabre S, di Clemente N.J Clin Endocrinol Metab. 2021 PMID: 33247926
The Goto-Kakizaki rat is a spontaneous prototypical rodent model of polycystic ovary syndrome. Bourgneuf C, Bailbé D, Lamazière A, Dupont C, Moldes M, Farabos D, Roblot N, Gauthier C, Mathieu d'Argent E, Cohen-Tannoudji J, Monniaux D, Fève B, Movassat J, di Clemente N, Racine C. Nat Commun. 2021 PMID: 33594056
Impact of sleep on female and male reproductive functions: a systematic review. Caetano G, I Bozinovic, C. Dupont, D. Léger, R. Lévy, N. Sermondade, Fertil Steril, 2021. PMID: 33054981
Exploring the potential impact of nutritionally actionable genetic polymorphisms on idiopathic male infertility: a review of current evidence. S. Mahbouli, C. Dupont, Y. Elfassy, E. Lameignère, R. Levy. Asian Journal of andrology. 2021. PMID: 33533736
New anti-Müllerian hormone target genes involved in granulosa cell survival in women with polycystic ovary syndrome. Racine C, Genêt C, Bourgneuf C, Dupont C, Plisson-Petit F, Sarry J, Hennequet-Antier C, Vigouroux C, Mathieu d'Argent E, Pierre A, Monniaux D, Fabre S, di Clemente N. J Clin Endocrinol Metab. 2020. PMID: 33247926
Relationships between metabolic status, seminal adipokines, and reproductive functions in men from infertile couples. Elfassy, Y ; Bongrani, A ; Levy, P ; Foissac, F ; Fellahi, S ; Faure, C McAvoy C, Capeau J, Dupont J, Fève B, Levy R, Bastard JP; Metasperme group. Eur J Endocrinol 2020. PMID: 31705791
Effectiveness of a therapeutic multiple-lifestyle intervention taking into account the periconceptional environment in the management of infertile couples: study design of a randomized controlled trial – The PEPCI study. Dupont C, Aegerter A, Foucaut AM, Reyre A, Lhuissier F, Bourgain M, Chabbert-Buffet N, Cédrin-Durnerin I, Selleret L, Cosson E and Lévy R. BMC pregnancy and childbirth. 2020. PMID: 32456614
Embryotoxicity Testing of IVF Disposables: How Do Manufacturers Test? Delaroche L, Oger P, Genauzeau E, Meicler P, Lamazou F, Dupont C, Humaidan P. Human Reproduction. 2020. PMID: 32053198
Prenatal programming by testosterone of follicular theca cell functions in ovary. Monniaux D, Genêt C, Maillard V, Jarrier P, Adriaensen H, Hennequet-Antier C, Lainé AL, Laclie C, Papillier P, Plisson-Petit F, Estienne A, Cognié J, di Clemente N, Dalbies-Tran R, Fabre S. Cell Mol Life Sci. 2020. PMID: 31327046
Persistent Müllerian duct syndrome due to anti-Müllerian hormone receptor 2 microdeletions: a diagnostic challenge. Tosca L, Giltay JC, Bouvattier C, Klijn AJ, Bouligand J, Lambert AS, Lecerf L, Josso N, Tachdjian G, Picard JY. Hum Reprod. 2020. PMID: 32187366
Aberrant granulosa cell-fate related to inactivated p53/Rb signaling contributes to granulosa cell tumors and to FOXL2 downregulation in the mouse ovary. Cluzet V, Devillers MM, Petit F, Chauvin S, François CM, Giton F, Genestie C, di Clemente N, Cohen-Tannoudji J, Guigon CJ. Oncogene. 2020. PMID: 31745296
INSERM
Kourilsky Building
34 rue Crozatier - 75012 Paris
France
Sorbonne Université Medicine
Saint-Antoine Site
27 rue Chaligny - 75012 Paris
France
